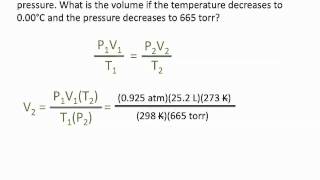
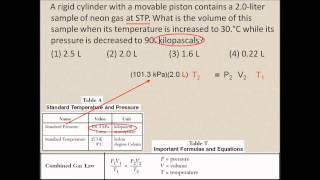
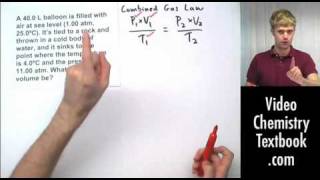
7. Gases
Chemistry Gas Laws: Combined Gas Law
7. Gases
Chemistry Gas Laws: Combined Gas Law
Additional 5 creators.
Learn with other creators
Showing 8 of 8 videos
Practice this topic
- Multiple Choice
A 4.30 L gas has a pressure of 7.0 atm when the temperature is 60.0 ºC. What will be the temperature of the gas mixture if the volume and pressure are decreased to 2.45 L and 403.0 kPa respectively?
883views2rank1comments - Multiple Choice
A sealed container with a movable piston contains a gas with a pressure of 1380 torr, a volume of 820 mL and a temperature of 31°C. What would the volume be if the new pressure is now 2.83 atm, while the temperature decreased to 25°C?
971views7rank4comments - Open Question
A sample of gas initially has a volume of 859 ml at 565 k and 2.20 atm. What pressure will the sample have if the volume changes to 268 mL while the temperature is increased to 815 K?
297views - Open Question
What is the temperature of an 11.2-L sample of carbon monoxide, CO, at 744 torr if it occupies 13.3 L at 55°C and 744 torr?
288views - Multiple ChoiceA 75.0 g sample of dinitrogen monoxide (N2O) is confined in a 4.12 L vessel. What is the pressure (in atm) at 205.0°F? Use the combined gas law equation and assume ideal gas behavior.
- Multiple ChoiceA 75.0 g sample of dinitrogen monoxide (N2O) is confined in a 4.12 L vessel. What is the pressure (in atm) at 205°F? Use the combined gas law equation to find the pressure.