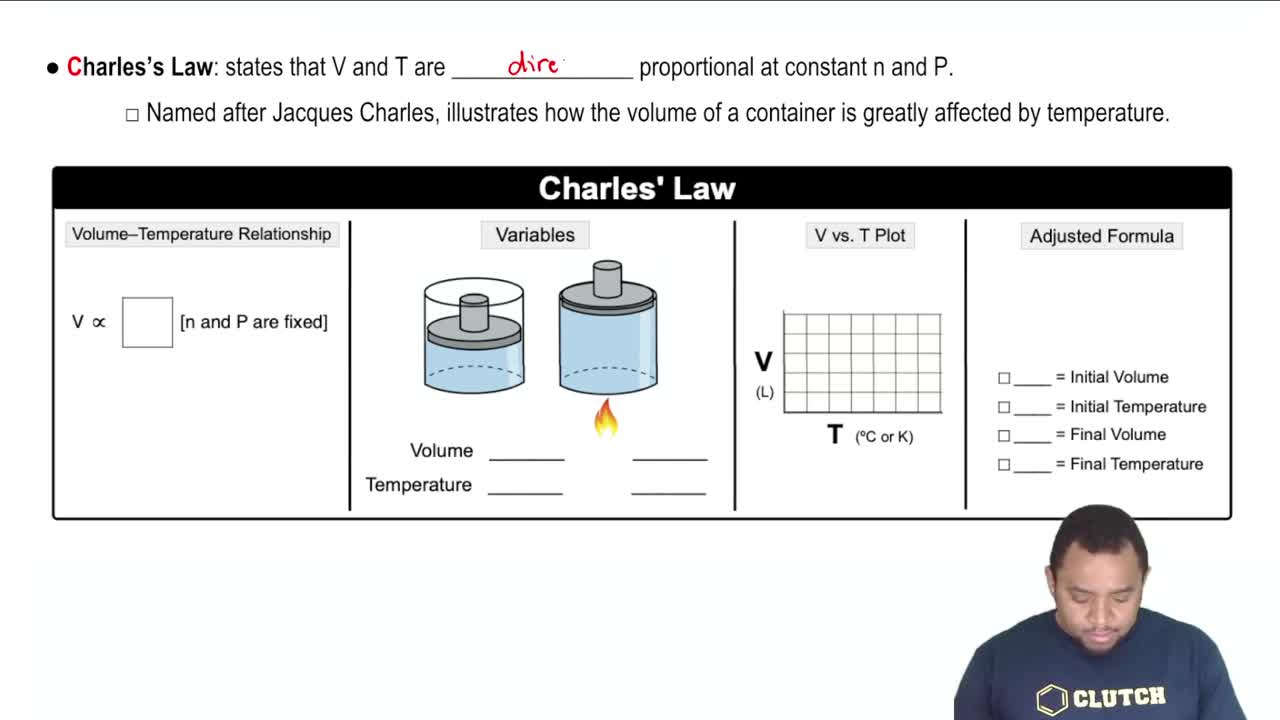
7. Gases
Chemistry Gas Laws
7. Gases
Chemistry Gas Laws
Showing 6 of 6 videos
Additional 6 creators.
Learn with other creators
Showing 9 of 9 videos
Practice this topic
- Multiple Choice
A 10.0 L cylinder with a movable piston exerts 3.00 atm of pressure. What will happen to the pressure if the volume of the container increases to 20.0 L?
1026views4rank - Multiple Choice
A sealed container with a movable piston contains a gas with a pressure of 1380 torr, a volume of 820 mL and a temperature of 31°C. What would the volume be if the new pressure is now 2.83 atm, while the temperature decreased to 25°C?
971views7rank4comments - Multiple ChoiceThe gas law that states that the volume of a gas is directly proportional to its amount in moles under conditions of constant pressure and temperature is known as1242views
- Open QuestionAvogadro's law states that at a given temperature and pressure, what quantity is constant?540views
- Open Question
Which formula represents Gay-Lussac's law? P1V1 = P2V2 P1T1 = P2T2
385views - Open Question
Which law can be used to calculate the number of moles of a contained gas?
300views - Multiple ChoiceA sample of gas has an initial volume of 5.6 L at a pressure of 735 mmHg. If the volume of the gas is increased to 9.4 L, what is its pressure, assuming temperature and amount of gas remain constant?