6. Chemical Quantities & Aqueous Reactions
Solutions
6. Chemical Quantities & Aqueous Reactions
Solutions
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple ChoiceWhat is the molar concentration of a solution prepared by dissolving 25.75 g of NaOH in water to produce 500.0 mL of solution?465views
- Multiple ChoiceAqueous solutions of sodium thiosulfate, Na2S2O3, are used as an antidote to some snakebites. How many moles of sodium ions are found in 10.0 mL of a 0.15 M solution of Na2S2O3?505views
- Multiple ChoiceHow many mL of 5.0 M nitric acid (HNO3) are required to make 1.00 L of a 0.00100 M solution of HNO3 (approximately the concentration of acid in acid rain)?415views1rank
- Open Question
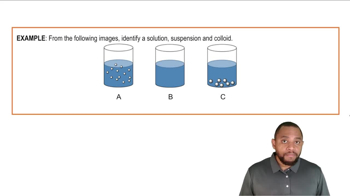
A(n) _____ is an unstable physical mixture of undissolved particles in a liquid.
359views - Open Question
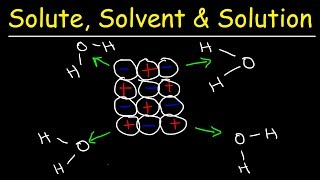
When 1 g of fructose is dissolved in 500 ml of water, is the solvent and is the solute.
357views - Open Question
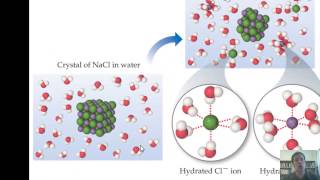
If you were to stir salt in water until the salt disappeared, which is the solute?
255views - Multiple ChoiceA solution is prepared by dissolving 28.4 g of glucose (C6H12O6) in 355 g of water. The final volume of the solution is 378 mL. What is the molarity of the solution?3views