6. Chemical Quantities & Aqueous Reactions
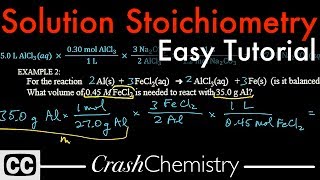
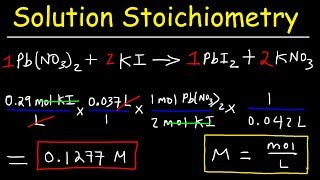
Solution Stoichiometry
Learn with other creators
Practice this topic
- Multiple Choice
How many milliliters of 0.325 M HCl are needed to react with 16.2 g of magnesium metal?
2 HCl (aq) + Mg (s) → MgCl2 + H2 (g)
1680views5rank2comments - Multiple Choice
What is the molar concentration of a hydrobromic acid solution if it takes 34.12 mL of HBr to completely neutralize 82.56 mL of 0.156 M Ca(OH)2?
2 HBr (aq) + Ca(OH)2 (aq) → CaBr2 (aq) + 2 H2O (l)
1126views3rank2comments - Multiple Choice
Consider the following balanced chemical equation:
H2O+ 2 MnO4– + 3 SO32- → 2 MnO2 + 3 SO42-+ 2 OH–
How many grams of MnO2 (MW:86.94 g/mol) will be created when 25.0 mL of 0.120 M MnO4– (MW:118.90 g/mol) reacts with 32.0 mL of 0.140 M SO32- (MW:80.07 g/mol).
857views2rank - Open Question
Titration of a 20.0-ml sample of acid rain required 1.7 ml of 0.0811 m NaOH to reach the end point. If we assume that the acidity of the rain is due to the presence of sulfuric acid, what was the concentration of sulfuric acid in this sample of rain?"
443views - Open QuestionWhat mass of na2cro4 is required to precipitate385views
- Open QuestionWhat mass of barium sulfate can be produced when350views
- Open Question
The mass percent of CaCO3(s) in the eggshell sample is closest to
609views - Multiple Choice100.0 mL of 0.100 M sodium phosphate is mixed with 100.0 mL of 0.120 M calcium chloride. What mass of calcium phosphate precipitate is formed?