6. Chemical Quantities & Aqueous Reactions
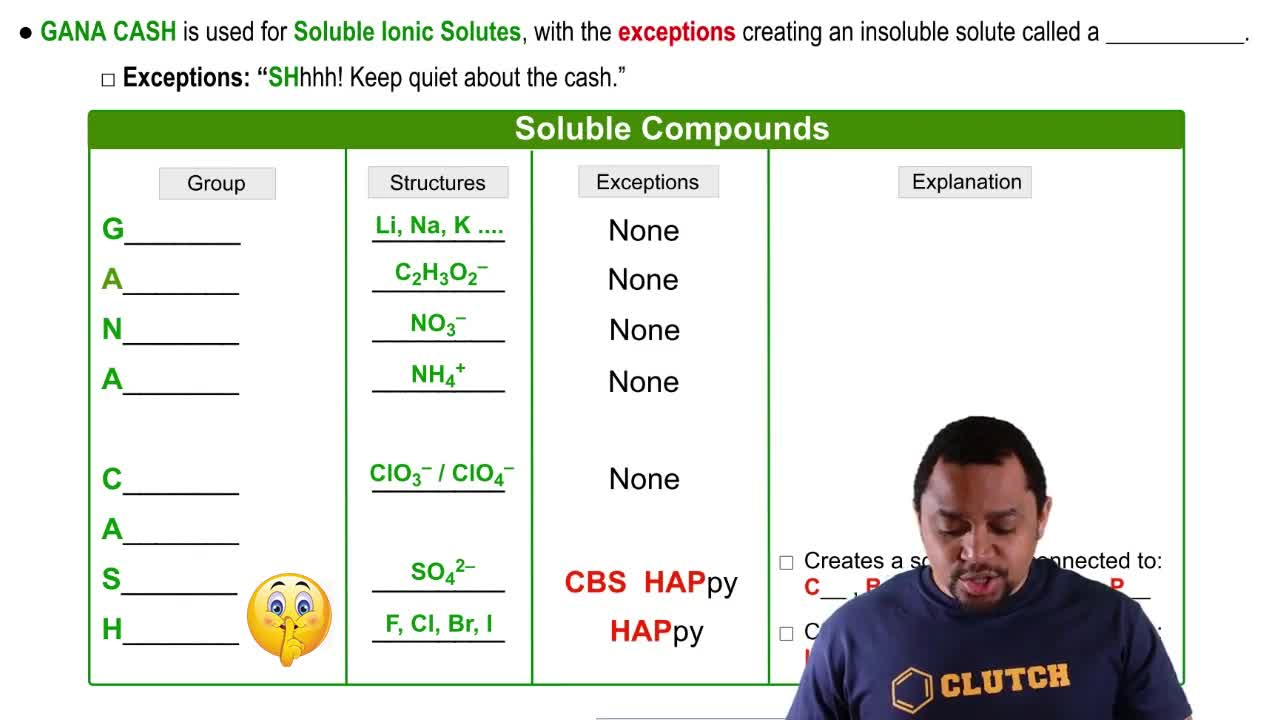
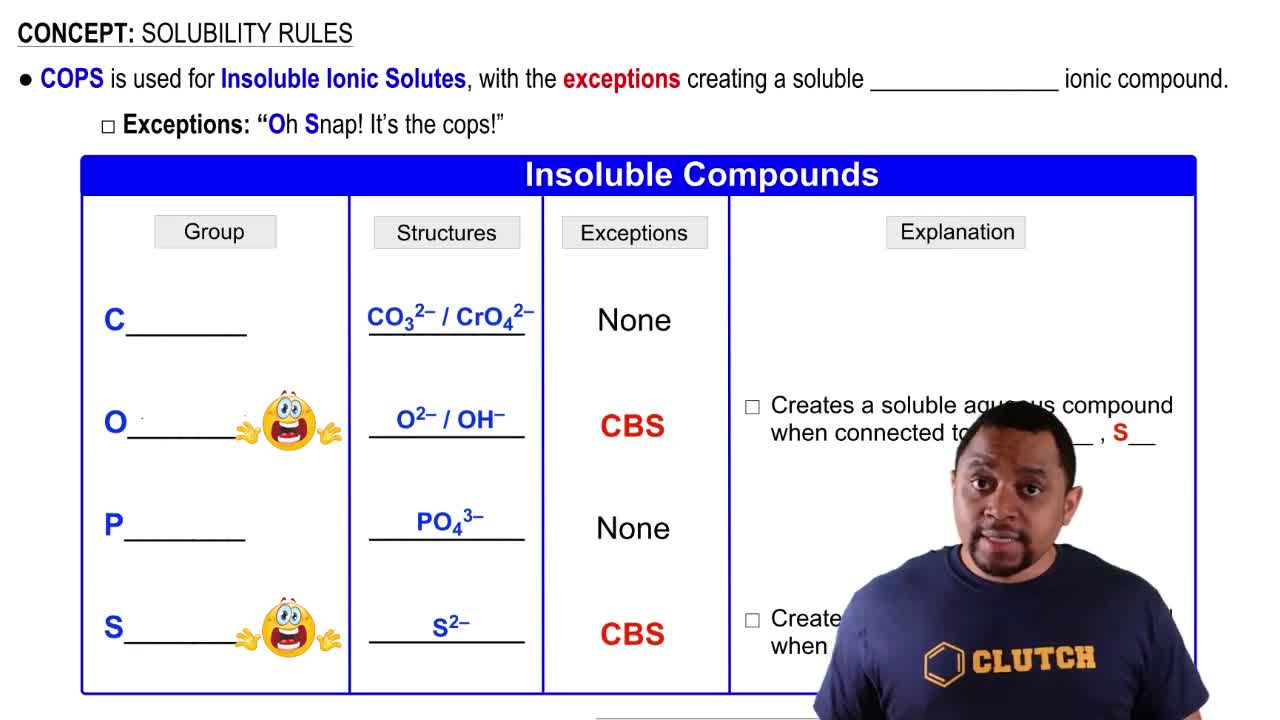
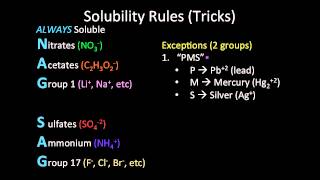
Solubility Rules
6. Chemical Quantities & Aqueous Reactions
Solubility Rules
Showing 7 of 7 videos
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
Based on your understanding of the solubility rules, which of the following ionic compounds will be insoluble?
1483views5rank - Multiple Choice
Which pair of compounds is insoluble in water?
2013views8rank3comments - Multiple ChoiceHard water often contains dissolved [Mg]2+ ions. Some laundry detergents contain sodium phosphate to soften hard water and to control acidity, thus making the detergent more effective.
What solid product forms when aqueous solutions of sodium phosphate and magnesium chloride are combined?398views - Multiple ChoiceIn which of the following types of chemical equations will the spectator ions be included?377views
- Open Question
What dissolved species are present in a solution of KCN?
266views - Open Question
What solute particles are present in an aqueous solution of CH3COCH3?
285views1rank - Open Question
Which of the schematic drawings best describes a solution of Li2SO4 in water? (for simplicity, the water molecules are not shown.)
372views - Open Question
When potassium chromate, K2CrO4 is dissolved in water, what ions are produced?
369views