6. Chemical Quantities & Aqueous Reactions
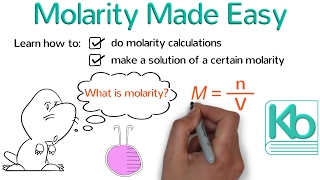
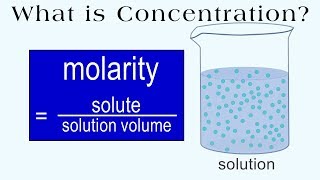
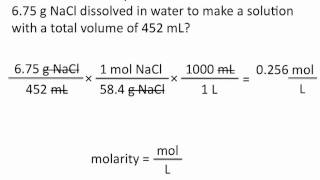
Molarity
Learn with other creators
Practice this topic
- Multiple Choice
What volume in (µL) of 0.125 M HBr contains 0.170 moles HBr?
1169views9rank1comments - Multiple Choice
Hypernatremia is a medical condition where a patient has high levels of sodium in their blood, and is the result of the body containing too little water. A patient has a measured sodium level of 165 mM. If 30.0 mL of their blood were drawn, what mass (in ng) of sodium would be present?
1049views5rank4comments - Multiple Choice
2.64 grams of an unknown compound was dissolved in water to yield 150 mL of solution. The concentration of the solution was 0.075 M. What was the molecular weight of the substance?
1086views4rank2comments - Multiple Choice
A solution with a final volume of 750.0 mL was prepared by dissolving 30.00 mL of benzene (C6H6, density = 0.8787 g/mL) in dichloromethane. Calculate the molarity of benzene in the solution.
1201views1rank3comments - Open Question
Determine the molarity of a solution formed by dissolving 97.7 g LiBr in enough water to yield 750.0 mL of solution.
249views - Open Question
What is the molarity of a solution that contains 17 g of NH3 in 0.50 L of solution?
300views - Open Question
Commercial grade hcl solutions are typically 39.0% (by mass) hcl in water. Determine the molarity of the HCl, if the solution has a density of 1.20 g/mL.
258views - Open Question
Find the molarity of a 40.0% by mass aqueous solution of sulfuric acid, H2SO4, for which the density is 1.3057 g/ml.
294views