6. Chemical Quantities & Aqueous Reactions
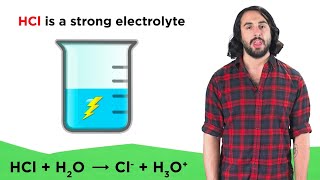
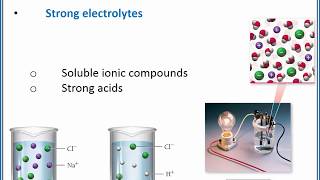
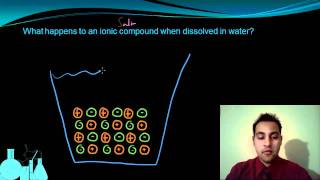
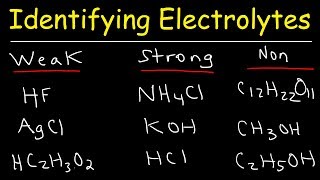
Electrolytes
Learn with other creators
Practice this topic
- Multiple Choice
Which of the following represents a strong base?
784views6rank1comments - Multiple Choice
Which of the following represents a non-electrolyte?
1283views3rank - Multiple Choice
Each of the following reactions depicts a solute dissolving in water. Classify each solute as a strong electrolyte, a weak electrolyte or a non-electrolyte.
a) PbSO4 (s) → PbSO4 (aq)
b) HC2H3O2 (aq) ⇌ H+ (aq) + C2H3O2− (aq)
c) CaS (s) → Ca2+ (aq) + S2− (aq)
d) Hg (l) → Hg (aq)
873views3rank - Multiple Choice
Which of the following statements is true?
765views6rank - Open Question
Which substance will most likely ionize when it is dissolved in water: HBr, CaBr2, MgCl2, or KCl?
373views - Open Question
How does a conductivity apparatus test whether a solution has ionic or covalent substances in it?
467views - Open Question
Which substance will most likely dissociate when it is dissolved in water? NO2, N2F6, CaCl2, C6H12O6
337views - Open Question
Which compound will conduct electricity when it is dissolved in water? CH4, CuSO4, C6H6, C6H12O6.
335views