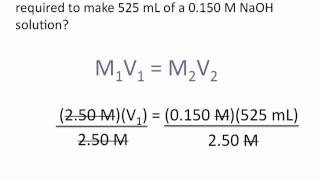6. Chemical Quantities & Aqueous Reactions
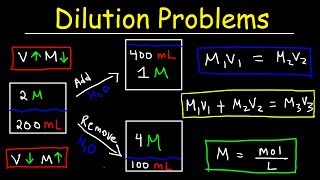
Dilutions
Learn with other creators
Practice this topic
- Multiple Choice
To what final volume would 100 mL of 5.0 M KCl have to be diluted in order to make a solution that is 0.54 M KCl?
938views8rank1comments - Multiple Choice
If 880 mL of water is added to 125.0 mL of a 0.770 M HBrO4 solution what is the resulting molarity?
882views7rank1comments - Multiple Choice
A student prepared a stock solution by dissolving 25.00 g of NaOH in enough water to make 150.0 mL solution. The student took 20.0 mL of the stock solution and diluted it with enough water to make 250.0 mL solution. Finally taking 75.0 mL of that solution and dissolving it in water to make 500 mL solution. What is the concentration of NaOH for this final solution? (MW of NaOH:40.00 g/mol).
1975views7rank5comments - Open Question
What is the concentration of a solution made by diluting 35 mL of 6.0 m HCl to a final volume of 750 mL?
367views - Open Question
What volume of 12.0 M HCl is required to make 75.0 mL of 3.50 M HCl?
286views - Open QuestionA standard solution is prepared for the analysis of fluoxymesterone411views
- Open Question
How much of a 0.250 M sucrose solution must be used to prepare 400.0 mL of a 0.0310 M solution? mL
323views - Multiple ChoiceA 100.0 mL sample is prepared from a 250.0 mL stock solution by pipetting 5.00 mL of the stock solution and diluting it until it had a molarity of 2.5 * 10^-4 M. What is the molarity of the original stock solution?