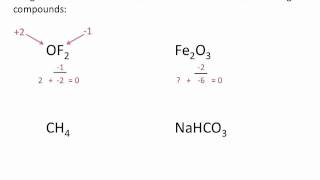6. Chemical Quantities & Aqueous Reactions
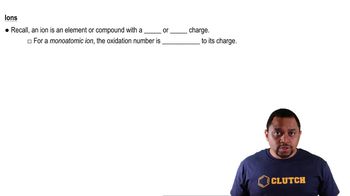
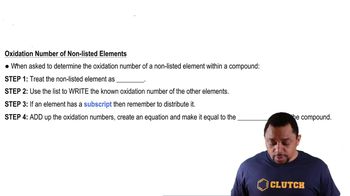
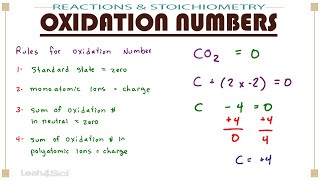
Calculate Oxidation Numbers
6. Chemical Quantities & Aqueous Reactions
Calculate Oxidation Numbers
Showing 8 of 8 videos
Additional 5 creators.
Learn with other creators
Showing 8 of 8 videos
Practice this topic
- Multiple Choice
Which of the following elements would have the lowest oxidation number?
919views10rank - Multiple ChoiceOxidation411views
- Multiple ChoiceIn which of the following does nitrogen have the largest positive value for its oxidation number?387views
- Open Question
What is the common oxidation state of the alkaline earth metals (Group 2)?
317views - Open Question
What is the oxidation state of each element in K2Cr2O7?
417views - Open Question
The total oxide ion charge in a formula unit of COO is 2–. What is the charge on each cobalt ion?
351views - Open Question
For the reaction KClO4 ⟶ KCl +2O2 assign oxidation numbers to each element on each side of the equation.
297views - Multiple ChoiceAssign an oxidation number to each atom in the compound K₂CrO₄.