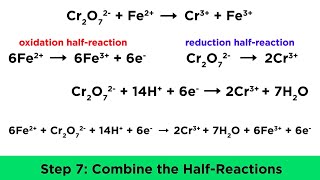
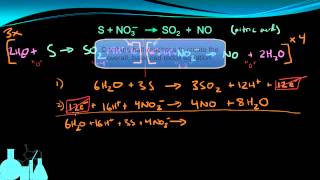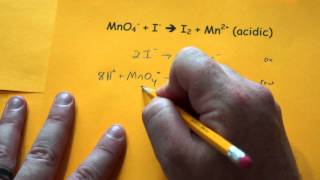6. Chemical Quantities & Aqueous Reactions
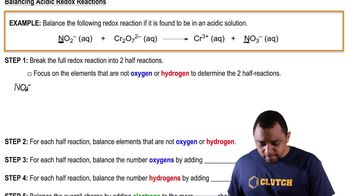
Balancing Redox Reactions: Acidic Solutions
6. Chemical Quantities & Aqueous Reactions
Balancing Redox Reactions: Acidic Solutions
Additional 2 creators.
Learn with other creators
Showing 5 of 5 videos
Practice this topic
- Multiple Choice
What is the coefficient of Fe3+ when the following reaction is balanced?
Bi3+ (aq) + Fe3+ (aq) + H2O (l) → BiO3- (aq) + Fe2+ (aq) + H+ (aq)
1081views1rank - Multiple ChoicePhosphoric acid H3PO4 has many industrial uses and is often found in some cola drinks. If you had 25.0 mL of a 0.200 M solution of H3PO4, how many mL of a 0.400 M solution of Ba(OH)2 would be required to neutralize the acid according to the following reaction? ?
2 H3PO4 (aq) + 3 Ba(OH)2 (aq) → Ba3(PO4)2 (s) + 6 H2O (l)
567views - Multiple ChoiceA 10.00-mL sample of a 0.130 M H3PO4 solution is added to 13.00 mL of a 0.150 M Ba(OH)2 solution. When the reaction is complete, what spectator ions are present?
2 H3PO4 (aq) + 3 Ba(OH)2 (aq) → Ba3(PO4)2 (s) + 6 H2O (l)
334views - Multiple ChoiceA 25.00-mL sample of H2SO4 solution of unknown concentration requires 28.27 mL of a 0.185 M KOH solution to complete the neutralization reaction. What is the concentration of the unknown H2SO4 solution?359views
- Open Question
Write a balanced overall reaction from these unbalanced half-reactions. Cu ⟶ Cu2+ Ag+ ⟶ Ag
295views1comments - Open QuestionComplete and balance the following half-reaction in acidic solution695views
- Open Question
Which is an important step in the alternate method for balancing equations in redox reactions?
322views - Open Question
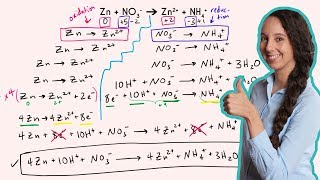
What is the oxidizing agent in the reaction Zn + 2 H+ → Zn2+ + H2?
436views