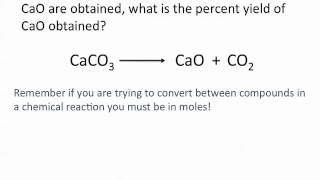
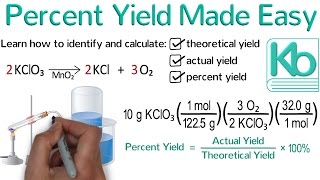
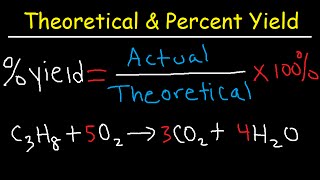
3. Chemical Reactions
Percent Yield
Learn with other creators
Practice this topic
- Multiple Choice
The reduction of iron (III) oxide creates the following reaction:
Fe2O3 (s) + 3 H2 (g) → 2 Fe (s) + 3 H2O (g)
If the above reaction only went to 75% completion, how many moles of Fe2O3 were require to produce 0.850 moles of Fe?
2134views7rank3comments - Multiple ChoiceGold metal can be reduced from the compound gold sulfide by hydrogen gas according to the following balanced chemical equation:
Au2S3 + 3 H2 → 3 H2S + 2 Au
How many grams of Au can be produced from 500.20 g of Au2S3 and 5.67 g H2?488views - Multiple ChoiceCyanogen (CN)2 has been observed in the atmosphere of Titan, Saturn’s largest moon, and in the gases of interstellar nebulas. On Earth, it is used as a welding gas and a fumigant. In its reaction with fluorine, carbon tetrafluoride (CF4) and nitrogen trifluoride (NF3) gases are produced:
(CN)2 + 7 F2 → 2 CF4 + 2 NF3
What is the percent yield of the reaction if 45.0 g of CF4 are isolated from the reaction between 75.0 g each of (CN)2 and F2?
437views - Multiple Choice
For the following chemical reaction 53.1 g HBrO4 is mixed with 25.8 g Li2CO3. Determine the percent yield if 7.17 g CO2 is produced.
2 HBrO4 (aq) + Li2CO3 (aq) → H2O (l) + CO2 (g) + 2 LiBrO4 (aq)
400views5rank - Open Question
What is the percent yield of NH3 if the reaction of 26.3 g of H2 produces 79.0 g of NH3?
288views - Open Question
What is the percent yield of HCl if 42.0 g of HCl are produced from the reaction of 62.0 g of PCl3?
298views