3. Chemical Reactions
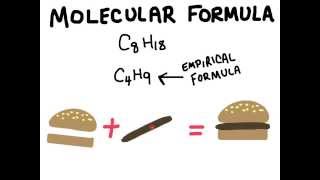
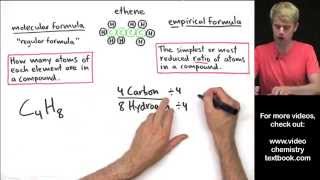
Molecular Formula
3. Chemical Reactions
Molecular Formula
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
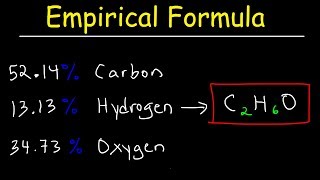
Elemental analysis of a pure compound indicated that the compound had 72.2% C, 8.50% H and the remainder as O. If 0.250 moles of the compound weighs 41.55 g, what is the molecular formula of the compound?
1121views18rank1comments - Multiple ChoiceWhich of the following is NOT a molecular compound?641views1rank
- Multiple ChoiceWhich of the following is a molecular element?464views
- Multiple ChoiceWhich of the following names and formulas are NOT paired correctly?351views
- Open Question
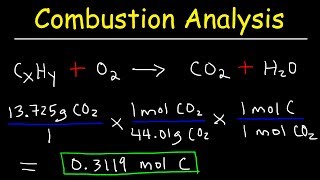
Dichloroethane, a compound that is often used for dry cleaning, contains carbon, hydrogen, and chlorine. it has a molar mass of 99 g/mol. analysis of a sample shows that it contains 24.3% carbon and 4.1% hydrogen. what is its molecular formula?
299views - Open Question
Find the molecular formula of each compound
266views - Open Question
Of the following molecular formulas for hydrocarbons which is an empirical formula
311views - Open Question
Elemental analysis of the unknown gas from part a revealed that it is 30.45% N and 69.55% O by mass. What is the molecular formula for this gas?
257views