3. Chemical Reactions
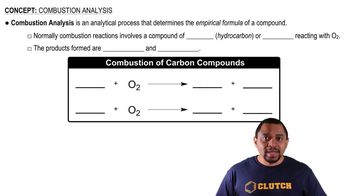
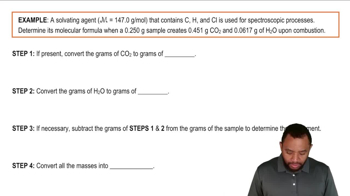
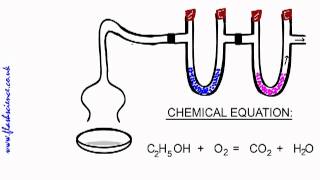
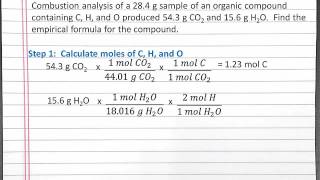
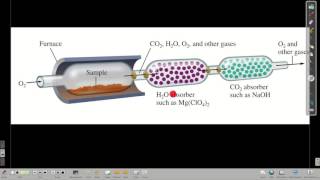
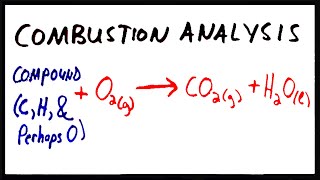
Combustion Analysis
Learn with other creators
Practice this topic
- Open Question
The combustion of 4.16 grams of a compound which contains only C,H,O and F yields 7.7 g CO2 and 2.52 g H2O. Another sample of the compound with a mass of 3.63 g is found to contain 0.58 g F. What is the empirical formula of the compound?
1493views15rank3comments - Multiple ChoiceWhen the following equation is balanced
C5H10(l) + O2(g) → CO2(g) + H2O(g)
What is the coefficient for carbon dioxide?413views - Multiple Choice
A compound composed of carbon, hydrogen and nitrogen undergoes a combustion reaction to produce 264.21 g CO2, 63.06 g H2O and 46.4 g NO2. Determine its empirical formula.
714views8rank - Multiple Choice
An unknown compound is composed of carbon, hydrogen and oxygen. A 4.30 g sample is ignited and creates 8.59 g CO2 and 3.52 g H2O. If the molar mass is 176.22 g/mol, what is the molecular formula?
503views5rank - Multiple Choice
In the presence of a small amount of oxygen a combustion reaction will not only produce carbon dioxide, but also carbon monoxide. The incomplete combustion of naphthalene, a hydrocarbon used in many dyes, produced 2.80 g CO, 4.40 g CO2 and 1.44 g H2O. Determine its empirical formula.
328views3rank1comments - Open Question
What else is produced during the combustion of propane, C3H8? H2O, C3H8, O2, C3H8O2.
505views - Open Question
Which clue can be used to identify a chemical reaction as a combustion reaction? Oxygen is a product. A hydrocarbon reacts with oxygen. Water and carbon dioxide react. The reaction involves ions.
286views - Open Question
Combustion analysis of 63.8 mg of a C, H and O containing compound produced 145.0 mg of co2 and 59.38 mg of H2O. what is the empirical formula for the
compound?
329views1rank