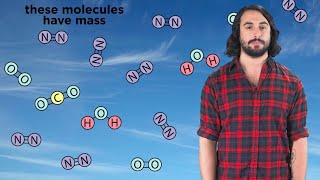
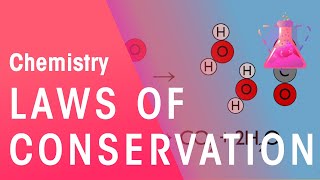
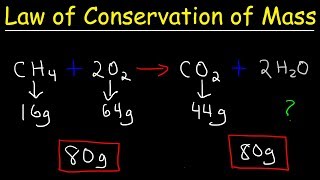
2. Atoms & Elements
Law of Conservation of Mass
Learn with other creators
Practice this topic
- Multiple Choice
Following the Law of Conservation of Mass, predict the minimum amount of nitrogen that will react with 50.0 grams of hydrogen to produce 92.5 grams of ammonia.
Nitrogen + Hydrogen → Ammonia
1253views15rank - Multiple Choice
Predict the amount of oxygen gas that will remain after the reaction of 112.6 grams of calcium with 24.0 grams of oxygen.
1043views17rank1comments - Multiple ChoiceThe law of conservation of mass states:575views
- Open Question
If the mass of the products measured 120 g, what would be the mass of the reactants?
366views - Open Question
Which best represents the law of conservation of mass?
a. mass of reactants > mass of products
b. mass of reactants < mass of products
c. mass of reactants = mass of products
d. mass of reactants → mass of products
434views - Open Question
Why does the mass of a substance remain the same after a chemical reaction?
374views - Open Question
The law of conservation of matter states that during a chemical reaction, the amount of matter
314views - Multiple ChoiceWhich process removes carbon dioxide from the atmosphere, demonstrating the Law of Conservation of Mass?