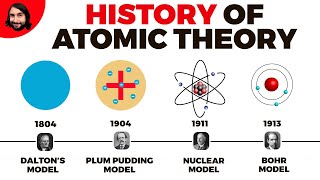
2. Atoms & Elements
Atomic Theory
2. Atoms & Elements
Atomic Theory
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
Which of the following is NOT a component of Dalton's Atomic Theory?
2657views10rank - Multiple Choice
Dalton used the lightest element as his standard for atomic mass. What is this element?
2394views14rank - Open Question
Who described atoms as small spheres that could not be divided into anything smaller?
1014views - Open Question
What experimental evidence led to the development of the current atomic model from the previous one?
783views - Open QuestionWhich of the following is not part of dalton's atomic theory?837views
- Open QuestionWhich chemical reactions are not possible according to dalton's atomic theory?1117views
- Multiple ChoiceWhich of the following statements about a carbon-12 atom is true?611views
- Multiple ChoiceWhich of the following statements is true regarding scientific theories in the context of atomic theory?316views