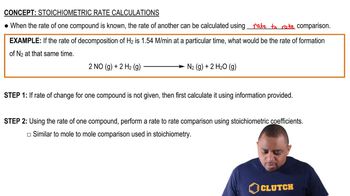
15. Chemical Kinetics
Stoichiometric Rate Calculations
15. Chemical Kinetics
Stoichiometric Rate Calculations
Additional 1 creators.
Learn with other creators
Practice this topic
- Multiple ChoiceUsing the following data, determine the order of the reaction with respect to [NO2–].
Data Table for Three Experiments Experiment Initial [NH4+] Initial [NO2] Initial rate (M/s) Experiment 1 0.24 M 0.10 M 7.2 × 10-6 M/s Experiment 2 0.12 M 0.10 M 3.6 × 10-6 M/s Experiment 3 0.12 M 0.15 M 5.4 × 10-6 M/s 486views - Multiple ChoiceUsing the data below (also from question 6), determine the overall order of the reaction.
Data Table for Three Experiments Experiment Initial [NH4+] Initial [NO2] Initial rate (M/s) Experiment 1 0.24 M 0.10 M 7.2 × 10-6 M/s Experiment 2 0.12 M 0.10 M 3.6 × 10-6 M/s Experiment 3 0.12 M 0.15 M 5.4 × 10-6 M/s 299views - Multiple ChoiceWrite the rate law for the following mechanism:
Step 1: NO2 + NO2 → NO3 + NO (slow)
Step 2: NO3 → NO + O2 (fast)
383views - Multiple Choice
The formation of alumina, Al2O3, can be illustrated by the reaction below:
4 Al (s) + 3 O2 (g) → 2 Al2O3 (s)
At 750 K it takes 267 seconds for the initial concentration of Al2O3 to increase from 6.18 x 10-5 M to 5.11 x 10-4 M. What is the average rate of Al?
169views3rank