15. Chemical Kinetics
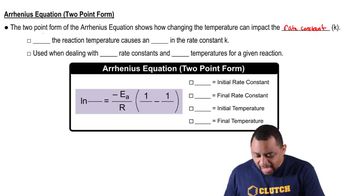
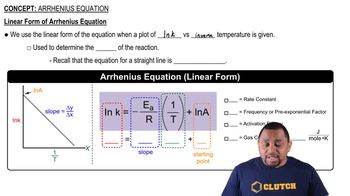
Arrhenius Equation
15. Chemical Kinetics
Arrhenius Equation
Showing 6 of 6 videos
Additional 1 creators.
Learn with other creators
Practice this topic
- Multiple Choice
The rate constant of a reaction at 32°C is 0.060/s. If the frequency factor is 3.1 × 1015 s–1, what is the activation barrier?
1417views2rank3comments - Multiple Choice
A reaction with an activation energy Ea = 55.00 kJ/mol is run at temperature of 30ºC. Determine the temperature required to increase the rate constant 3 times.
1264views2rank4comments - Multiple Choice
The following data shows the rate constant of a reaction measured at numerous temperatures. Use the Arrhenius plot to determine the frequency factor for the reaction.
4554views2rank6comments - Open Question
The rate of a certain reaction was studied at various temperatures. The table shows temperature (𝑇) and rate constant (𝑘) data collected during the experiments. Plot the data to answer the questions. What is the value of the activation energy, 𝐸a, for this reaction?
542views - Multiple ChoiceA certain first-order reaction has a rate constant of 2.63 x 10^-2 s^-1 at 22.0°C. What is the value of the rate constant k at 75.0°C if the activation energy Ea is 76.9 kJ/mol?