14. Solutions
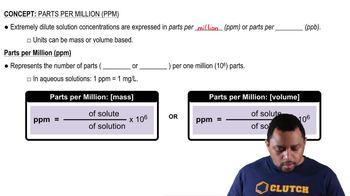
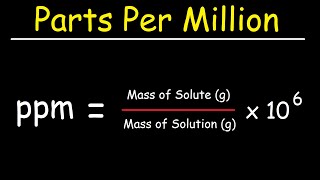
Parts per Million (ppm)
14. Solutions
Parts per Million (ppm)
Additional 2 creators.
Learn with other creators
Showing 5 of 5 videos
Practice this topic
- Multiple Choice
A 5.12 L sample of solution contains 0.230 g of potassium sulfate, K2SO4. Determine the concentration of K2SO4 in ppm if the density of the solution is 1.30 g/mL.
1021views4rank1comments - Multiple Choice
Calculate the concentration in parts per billion of the following aqueous solution:0.91 mg of caffeine in a total volume of 131 mL.
1746views5rank2comments - Multiple Choice
Glucose makes up about 0.102% by mass of human blood. Calculate this concentration in ppm.
1360views4rank - Multiple Choice
The average human body contains about 5,000 grams of blood. What mass of arsenic is present in the body if the amount in blood is 0.86 ppb?
4319views1comments