14. Solutions
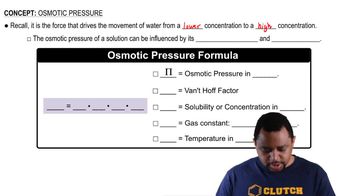
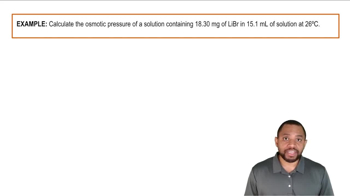
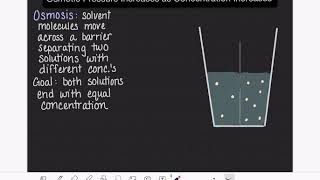
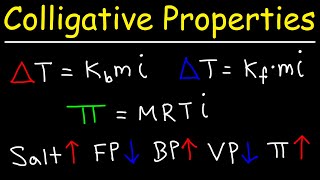
Osmotic Pressure
Learn with other creators
Practice this topic
- Multiple Choice
The osmotic pressure of blood is 5950.8 mmHg at 41°C. What mass of glucose, C6H12O6, is needed to prepare 5.51 L of solution. The osmotic pressure of the glucose solution is equal to the osmotic pressure of blood.
1836views6rank - Multiple ChoiceDetermine the osmotic pressure at 22.5 °C of a 2.5 L aqueous solution that contains 50.0 g of sucrose (molar mass = 342.3).998views
- Multiple ChoiceWhich of the following characterizes a colloid?985views
- Open Question
A 150.0 mL sample of an aqueous solution at 25°C contains 15.2 mg of an unknown nonelectrolyte compound. if the solution has an osmotic pressure of 8.44 torr, what is the molar mass of the unknown compound?
968views1rank - Open Question
Intravenous, or IV, solutions used in medicine must exert the same osmotic pressure as blood to prevent a net flow of water into or out of the blood cells. the proper concentration for an intravenous NaCl solution is 0.90 g NaCl per 100. ml of solution (sometimes referred to as 0.90% m/v). If the van't hoff factor of NaCl is 𝑖=1.8, what is the osmotic pressure of blood at body temperature, 37∘C?
1069views - Open Question
What is the osmotic pressure of a 0.850 M solution of glucose in water at 35 °C?
1017views - Multiple Choice
The osmotic pressure of a solution containing 7.0 g of insulin per liter is 23 torr at 25ºC. What is the molar mass of insulin? (1 atm = 760 torr)
744views2comments