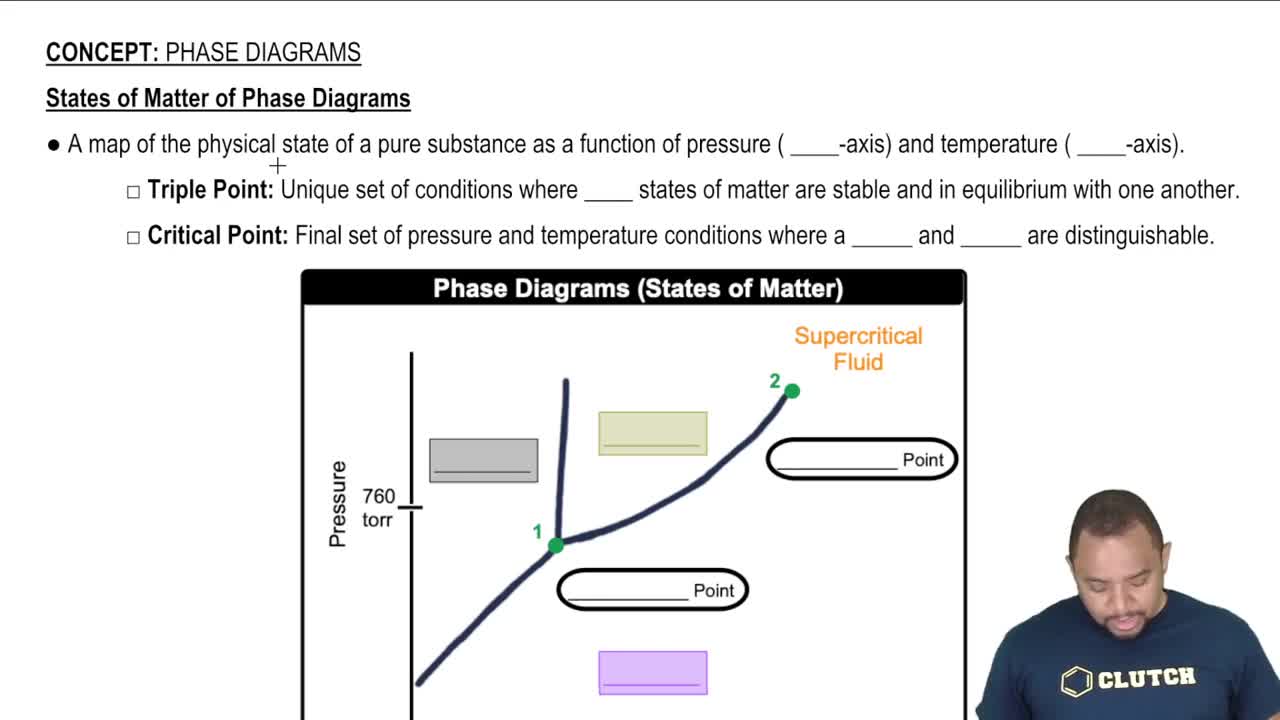
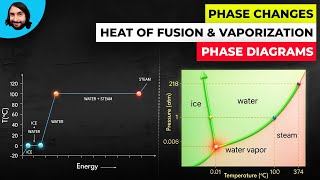
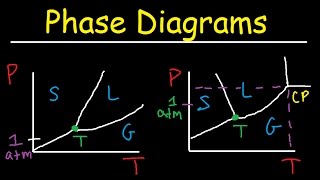
13. Liquids, Solids & Intermolecular Forces
Phase Diagrams
13. Liquids, Solids & Intermolecular Forces
Phase Diagrams
Additional 5 creators.
Learn with other creators
Showing 8 of 8 videos
Practice this topic
- Multiple Choice
The critical point of this substance occurs at what temperature?
1143views4rank - Multiple Choice
Arrow I corresponds to:
835views1rank1comments - Multiple Choice
What is the normal freezing point of this unknown substance?
2048views5rank - Multiple Choice
At what temperature can we no longer tell the difference between the liquid and gas phases?
910views9rank - Open Question
Based on the phase diagram shown below, how will the melting point of the substance change if the pressure is increased above 1 atm?
485views - Open Question
In the phase diagram for water, indicate the direction that the solid–liquid and liquid–gas coexistence lines will move along the temperature axis after the addition of solute.
575views - Open Question
A gaseous substance turns directly into a solid. Which term describes this change?
374views - Open Question
The boiling temperature and its relationship to the system exist in the ____.
479views