13. Liquids, Solids & Intermolecular Forces
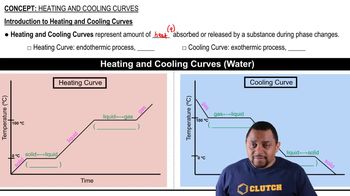
Heating and Cooling Curves
13. Liquids, Solids & Intermolecular Forces
Heating and Cooling Curves
Showing 5 of 5 videos
Additional 2 creators.
Learn with other creators
Showing 5 of 5 videos
Practice this topic
- Multiple Choice
How much energy (kJ) is required to convert a 76.4 g acetone (MM = 58.08 g/mol) as a liquid at -30°C to a solid at -115.0°C?
1323views4comments - Multiple Choice
If 53.2kJ of heat are added to a 15.5g ice cube at - 5.00 oC, what will be the resulting state and temperature of the substance?
1552views5rank5comments - Multiple ChoiceCalculate the volume (mL) of acetone, C3 H6 O, (density = 0.786 g/mL) that can be vaporized at its normal boiling point with 345 kJ of heat. ΔHvap for acetone is 29.1 kJ/mol.710views
- Multiple ChoiceDetermine the vapor pressure (atm) of rubbing alcohol (isopropanol) at 20.0℃. The normal boiling point of isopropanol is 82.3℃ and the heat of vaporization (ΔHvap) is 39.9 kJ/mol.722views
- Open Question
How much energy is released when 42.5 g of water freezes?
382views - Open Question
What quantity of heat, in kj, is required to convert 50.0 g of ethanol (C₂H₅OH) at 23.0°C to a vapor at 78.3°C (its boiling point)? (specific heat capacity of ethanol = 2.46 J/g • c; ∆Hvap = 39.3 kJ/mol)
396views - Open Question
Which is the process by which a gas changes to a solid: deposition, evaporation, freezing, or sublimation?
268views - Open Question
What are greenhouse gases? How does carbon dioxide enter the atmosphere? (Site 1)
271views