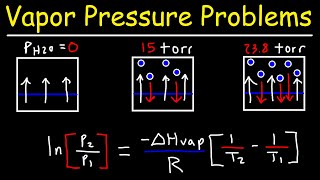
13. Liquids, Solids & Intermolecular Forces
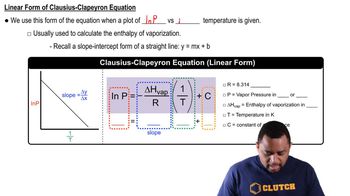
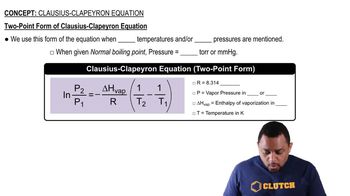
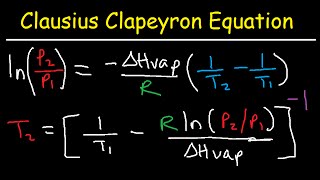
Clausius-Clapeyron Equation
13. Liquids, Solids & Intermolecular Forces
Clausius-Clapeyron Equation
Showing 5 of 5 videos
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
Vapor pressure measurements at various temperature values are given below. Determine the molar heat of vaporization for cyclohexane.
929views7rank1comments - Multiple Choice
Benzene has a heat of vaporization of 30.72 kJ/mol and a normal boiling point of 80.1°C. At what temperature does benzene boil when the external pressure is 405 torr?
5011views2rank4comments - Open Question
Which of the temperatures below is most likely to be the boiling point of water at 880 torr?
261views - Multiple ChoiceA substance has a normal boiling point of 76 °C and an enthalpy of vaporization (ΔvapH) of 38.7 kJ/mol. Using the Clausius-Clapeyron equation, determine the vapor pressure (in mbar) of this substance at 29 °C.
- Multiple ChoiceBenzene has a heat of vaporization of 30.72 kJ/mol and a normal boiling point of 80.1 °C. At what temperature does benzene boil when the external pressure is 577 mbar?1views