13. Liquids, Solids & Intermolecular Forces
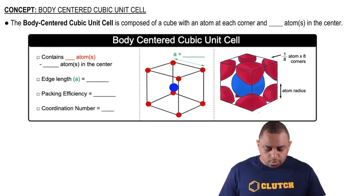
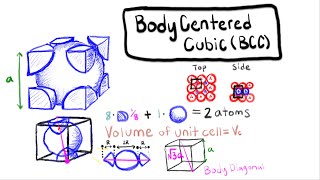
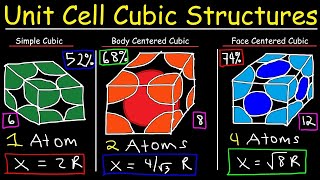
Body Centered Cubic Unit Cell
13. Liquids, Solids & Intermolecular Forces
Body Centered Cubic Unit Cell
Additional 2 creators.
Learn with other creators
Showing 5 of 5 videos
Practice this topic
- Multiple Choice
Tungsten possesses a body-centered cubic structure. If its density is 19.28 g/cm3, what is its radius in pm?
907views - Multiple Choice
Vanadium has a body-centered cubic structure. If the atomic radius of vanadium is 134 pm, calculate the density of solid vanadium.
1358views2rank - Multiple Choice
The edge of a body-centered cubic unit cell of an element Z was found to be 2.88 x 10-8 cm. The density of the element is 7.2 g/cm3. What is the approximate molar mass of Z?
950views3rank - Open Question
Vanadium atom has a radius of 131 pm and crystallizes with a bcc unit cell. Determine the number of unit cells present in 1.5 cm3 solid sample of vanadium.
348views - Open Question
Titanium metal (d = 4.50 g/cm3) has a body-centered cubic unit cell. Calculate the edge length of the unit cell in picometer. (1 pm = 10-12 m)
432views - Multiple ChoiceA metal crystallizes in a body-centered cubic structure with a unit cell edge length of 4.31 Å. What is the radius of the atoms in Å?1views