12. Molecular Shapes & Valence Bond Theory
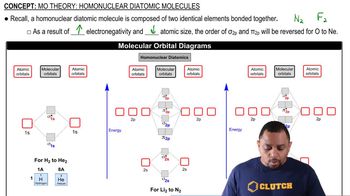
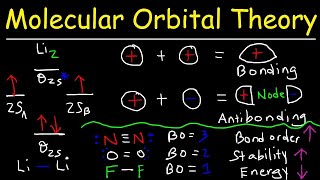
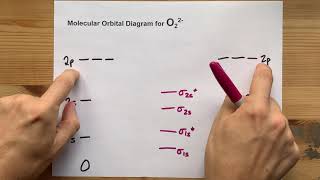
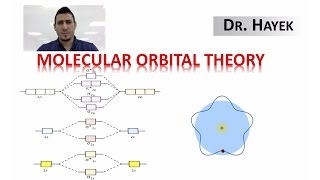
MO Theory: Homonuclear Diatomic Molecules
Learn with other creators
Practice this topic
- Multiple Choice
Determine the number of electrons found in the π2p orbitals for the dioxygen dication, O22+.
915views1rank - Multiple Choice
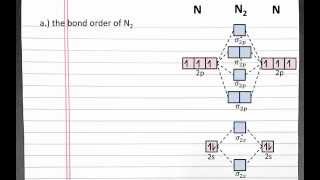
Using a MO diagram, write the electron configuration for the P2 molecule
4484views3rank1comments - Multiple ChoiceWhich of the following statements is true about polarity?857views
- Multiple ChoiceWhich of the following molecules is nonpolar?435views
- Open Question
Suppose that you have 16 diatomic molecules or ions with the valence molecular orbital arrangement shown here (figure 1), but with different numbers of valence electrons. species 1 has one valence electron, species 2 has two valence electrons, etc. classify each as diamagnetic or paramagnetic.
285views - Open Question
Construct the molecular orbital diagram for H2− .
275views - Open QuestionUse the molecular orbital diagram shown to determine which of the following are paramagnetic.868views
- Open Question
Suppose that you have 16 diatomic molecules or ions with the valence molecular orbital arrangement shown here (Figure 1), but with different numbers of valence electrons. Species 1 has one valence electron, Species 2 has two valence electrons, etc. Classify each as diamagnetic or paramagnetic.
903views