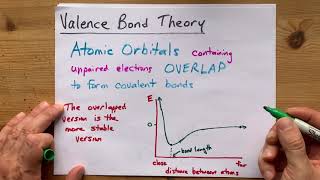
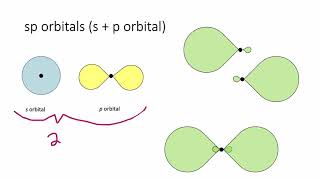
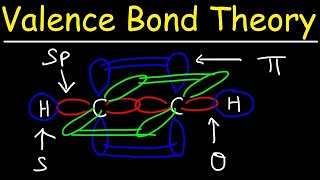
12. Molecular Shapes & Valence Bond Theory
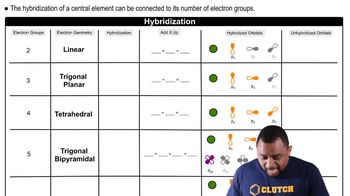
Hybridization
12. Molecular Shapes & Valence Bond Theory
Hybridization
Additional 2 creators.
Learn with other creators
Showing 5 of 5 videos
Practice this topic
- Multiple Choice
How many of the following molecules have sp3d2 hybridization on the central atom?
SeCl6 XeCl4 IF5 AsCl5
1743views6rank1comments - Multiple Choice
How many unhybridized orbitals does the beryllium atom possess in BeCl2?
1133views - Open Question
Draw and determine the hybridization and unhybridized orbitals for the following covalent compound.
KrBr4 Hybridization:
Unhybridized Orbitals:
2048views10rank - Multiple ChoiceDetermine the geometry around the central oxygen atom and the molecular polarity of dimethyl ether:419views
- Multiple ChoiceWhat is the hybridization of the nitrogen atom in ammonia?600views
- Open Question
Consider the structure of the amino acid alanine. Indicate the hybridization about each interior atom.
319views - Open QuestionWhat types of orbital overlap occur in cumulene?928views
- Open QuestionWhat is the hybridization of the central atom of each of the following molecules?368views