1. Intro to General Chemistry
Density
1. Intro to General Chemistry
Density
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
When lead levels in blood exceed 0.80 ppm (parts per million) the level is considered dangerous. 0.80 ppm means that 1 million g of blood would contain 0.80 g of Pb. Given that the density of blood is 1.060 kg/cm3, how many grams of Pb would be found in 400.00 mL of blood at 0.620 ppm?
1803views2rank13comments - Multiple ChoiceHow many mL of bromine are required to deliver 0.25 g of bromine into a reaction?
(dBr = 3.12 g/cm3)394views - Open Question
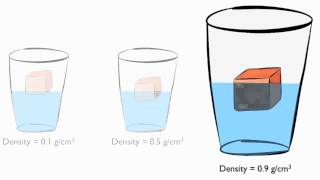
If a substance has a large mass and a small volume, what can you conclude about it?
294views - Open Question
What is the mass of 2.00 L of an intravenous glucose solution with a density of 1.15 g/mL?
469views - Open Question
What two quantities must be known to calculate the density of a sample of matter?
292views - Open Question
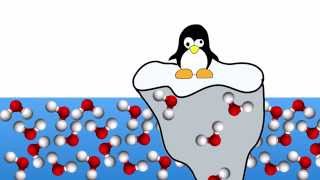
If the solid form of most molecules is heavier than the liquid form, why does ice float?
226views - Multiple ChoiceA block of solid that weighs 58.16 g was placed in a container with a 100 mL capacity. It was then filled to capacity with liquid benzene and measured to have a combined weight of 96.15 g. Calculate the density of the solid if the density of benzene is 0.879 g/mL.1views
- Multiple ChoiceA small flask and stopper is found to weigh 23.994 g. It is carefully filled with water and reweighed. The new weight is 50.00 g. If the water density is taken as 1.000 g/ml, what is the volume of the flask?1views