1. Intro to General Chemistry
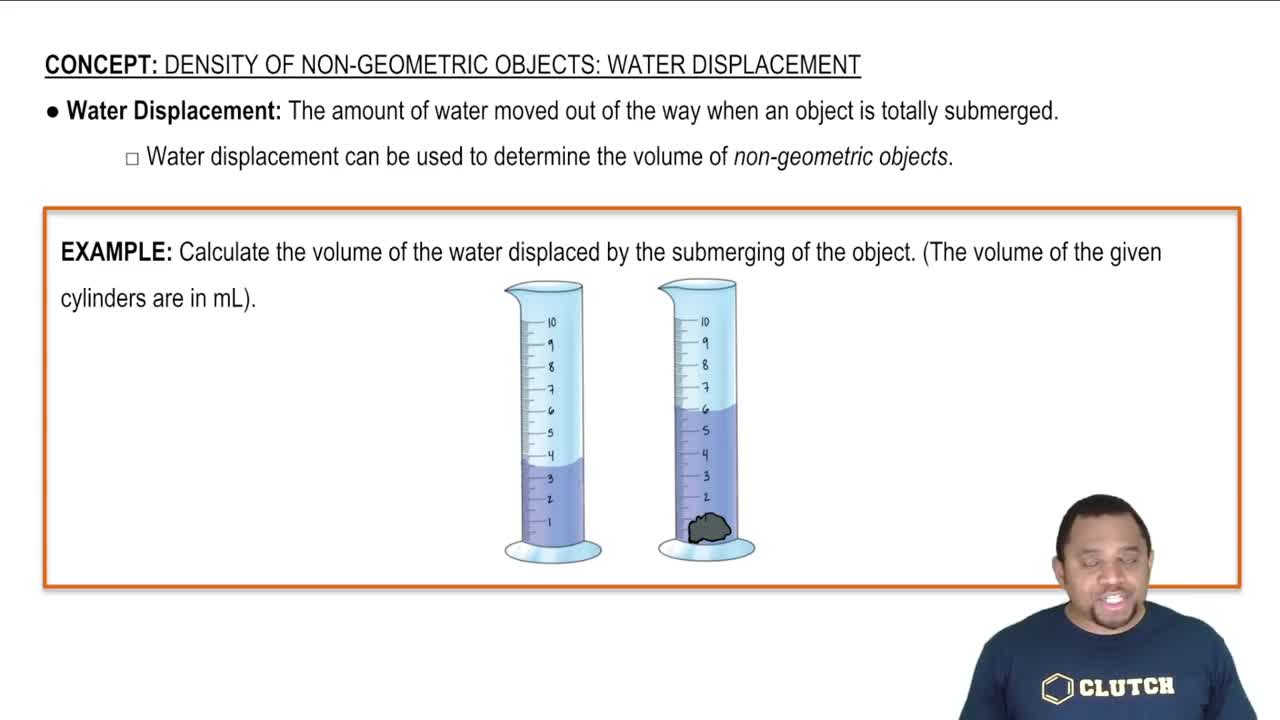
Density of Non-Geometric Objects
1. Intro to General Chemistry
Density of Non-Geometric Objects
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
A piece of unknown solid weighs approximately 0.045 lbs. When a scientist places it in a glass beaker the water level increases from 200 mL to 260 mL. What is the density of the unknown solid in g/mL?
1228views9rank2comments - Multiple Choice
If an irregularly shaped apple possesses a density of 0.96 g/cm3, what is its mass in milligrams? (The volume of the given cylinders are in mL).
1253views9rank2comments - Open Question
Determine the density of an object that has a mass of 149.8 g and displaces 12.1 mL of water when placed in a graduated cylinder.
363views - Open Question
Which property is used to determine if an object is made of pure silver (Ag)?
245views - Multiple ChoiceWhat is the density of FeCl2 if the mass of the empty volumetric flask is 550g and when the flask is filled with 1000ml of FeCl2 at 20°C, it has a mass of 2000g?1views
- Multiple ChoiceWhat is the total mass of the three ice cubes placed in the water, given that the density of ice is 0.92 g/cm³ and each cube has a side length of 3.0 cm?1views