24. Transition Metals and Coordination Compounds
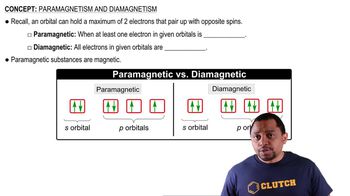
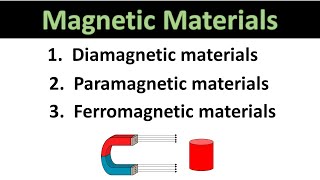
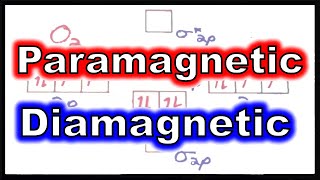
Paramagnetism and Diamagnetism
24. Transition Metals and Coordination Compounds
Paramagnetism and Diamagnetism
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
Which of the following atoms has the most unpaired electrons?
1649views5rank - Multiple Choice
Write the condensed electron configuration for the nickel (III) ion and state if it is paramagnetic or diamagnetic.
1152views7rank1comments - Multiple Choice
Write the condensed electron configuration for the copper (I) ion and is it magnetic?
802views6rank1comments - Multiple ChoiceWhich one of the following atoms or ions is diamagnetic?477views
- Open Question
In the ground-state electron configuration of Fe3+, how many unpaired electrons are present?
376views - Open QuestionSelect the element(s) that will have one unpaired electron in the p orbital.633views
- Open QuestionIdentify the general outer electron configuration for each group of elements shown in this periodic table outline.455views
- Open QuestionSort the following atom or ions as paramagnetic or diamagnetic according to the electron configurations determined in part a.398views