23. Chemistry of the Nonmetals
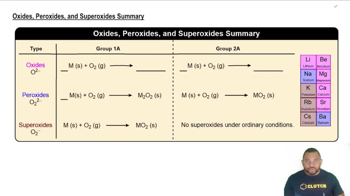
Oxides, Peroxides, and Superoxides
23. Chemistry of the Nonmetals
Oxides, Peroxides, and Superoxides
Showing 5 of 5 videos
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple ChoiceWhich of the compounds of sulfur listed below could be used as a dehydrating agent?277views
- Multiple ChoiceH2S is converted into elemental sulfur through the two-step process called the Claus process:
Step 1: 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 H2O (g)
Step 2: 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 H2O (g)Determine the number of electrons transferred in step one of the Claus process.
326views - Multiple ChoiceWhat are the oxidation states of oxygen in the following compounds?
K2O2 CaO CsO2
378views - Multiple Choice
Classify each of the following compounds as an oxide, peroxide, or superoxide.
i) Li2O _____________________
ii) RbO2 _____________________
iii) BaO2 _____________________
iv) SrO _____________________
271views