20. Electrochemistry
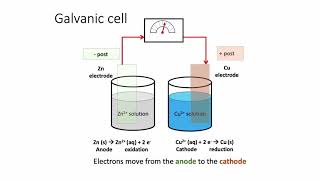
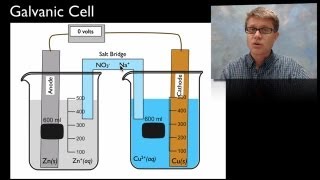
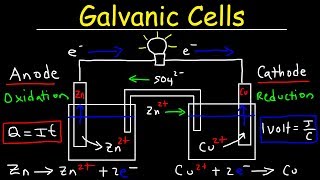
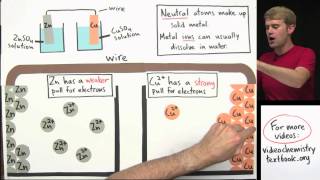
Galvanic Cell
20. Electrochemistry
Galvanic Cell
Showing 8 of 8 videos
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
Given the following standard reduction potentials,
Hg22+(aq) + 2 e– 2 Hg (l) E° = +0.789 V
Hg2Cl2(s) + 2 e– 2 Hg (l) + 2 Cl-(aq) E° = +0.271 V
determine Ksp for Hg2Cl2(s) at 25 °C.
1656views1rank9comments - Multiple ChoiceDetermine the standard cell potential for the following cell:
Ni (s)│Ni2+ (aq) ║Cu2+ (aq)│Cu (s) cell.
1049views - Multiple ChoiceIn a galvanic cell, the direction of the flow of electrons __________ .987views
- Multiple ChoiceWhich of the following correctly describes the products of the electrolysis of an aqueous solution of KI?889views
- Open Question
For the redox reaction label: the anode, cathode, half-reactions occurring at each half-cell, direction of electron flow, and direction of neutral ions flow.
1089views3rank1comments - Open Question
Most of today's EVs use ____________ batteries.
1031views - Open Question
A positive electrode is called a(n) _____.
791views - Open Question
What is the reduction half-reaction for the following overall galvanic cell reaction? Co2+(aq) + 2 Ag(s) → Co(s) + 2 Ag+(aq)
688views