20. Electrochemistry
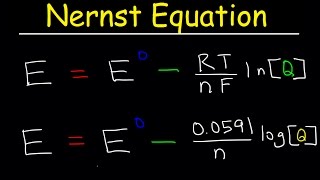
Cell Potential: The Nernst Equation
20. Electrochemistry
Cell Potential: The Nernst Equation
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
If [Br–] = 0.010 M and [Al3+] = 0.022 M, predict whether the following reaction would proceed spontaneously as written at 25ºC:
Al (s) + Br2 (l) ⇌ Al3+ (aq) + Br– (aq)
Standard Reduction Potentials
Al3+ (aq) + 3 e– → Al (s) E°red = –1.66 V
Br2 (l) + 2 e– → Br– (aq) E°red = +1.09 V
293views2rank1comments - Multiple Choice
Determine [Fe2+] for the following galvanic cell at 25ºC if given [Sn2+] = 0.072 M, [Fe3+] = 0.0219 M, and [Sn4+] = 0.00345 M.
Sn2+ (aq) + 2 Fe3+ (aq) ⇌. Sn4+ (aq) + 2 Fe2+ (aq) Ecell = + 0.68 V
Standard Reduction Potentials
Sn4+ (aq) + 2 e– →. Sn2+ (aq) E°red = + 0.151 V
Fe3+ (aq) + e– → Fe2+ (aq) E°red = + 0.771 V
379views1rank1comments - Open Question
______________________ accelerates the rusting process.
334views - Multiple ChoiceUsing the Nernst equation, calculate the concentration of Cu^2+ ions given the cell potential is 0.5540 V and the standard cell potential is 0.4260 V. The equation is: 0.5540 V = 0.4260 V - (0.0592/2) log ([Cu^2+]/[1]^2). What is the concentration of Cu^2+?
- Multiple ChoiceA voltaic cell is constructed with two Zn2+-Zn electrodes, where the half-reaction is Zn2+ + 2e- → Zn(s) with E° = -0.763 V. The concentrations of zinc ion in the two compartments are 5.50 M and 1.11 × 10^-2 M, respectively. What is the cell emf in volts?








![19.5 How to Calculate Nonstandard Cell Potential [Nernst Equation] | General Chemistry](https://img.youtube.com/vi/Ma0TC3V9bdI/mqdefault.jpg)