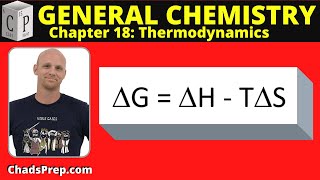
19. Chemical Thermodynamics
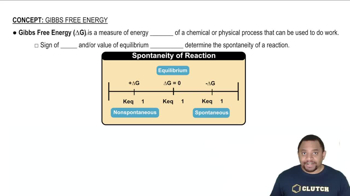
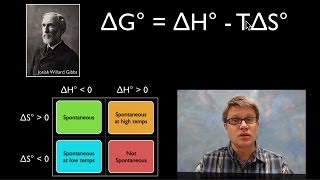
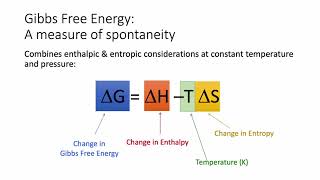
Gibbs Free Energy
19. Chemical Thermodynamics
Gibbs Free Energy
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
If ∆G is small and positive which of the following statements is true?
1278views2rank2comments - Multiple Choice
Nitrogen gas combines with fluorine gas to form nitrogen trifluoride according to the reaction below at 25oC:
N2 (g) + 3 F2 (g) → 2 NF3 (g) ΔHo = -249.0 kJ ΔSo = -278 J/K
Calculate ΔGo and state if the reaction favors reactants or products at standard conditions.
2694views4rank7comments - Multiple ChoiceCalculate ΔG (kJ/mol) for the following reaction at 298 K when PH2 = 0.0200 atm and PC3H8 = 0.500 atm.
3 C (s, graphite) + 4 H2 (g) → C3H8 (g)
400views - Multiple Choice
The chemical reaction 2 NO2Br (g) → 2 NO2 (g) + Br2 (g) has a Keq = 4.50 × 105.
Does the reaction increase the entropy of the Universe? Explain.
499views2rank - Open QuestionWhich one of the following is always positive when a spontaneous process occurs?408views
- Open QuestionWhich one of the following is always positive when a spontaneous process occurs467views
- Open Question
Reactions in which there is a negative change in free energy (–ΔG) are:
250views