19. Chemical Thermodynamics
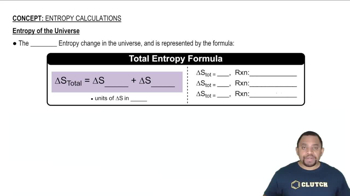
Entropy Calculations
19. Chemical Thermodynamics
Entropy Calculations
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
For the following reaction at 27 °C, calculate ∆S°rxn, ∆Ssurr, and ∆Stot. Determine if reaction is favorable.
Fe2O3 (s) + 3 H2 (g) → 2 Fe (s) + 3 H2O (g) ∆Hrxn = 98.8 kJ
352views2rank - Open Question
Find ΔrS for the formation of CH3Cl(g) from its elements in their standard states. standard thermodynamic quantities for selected substances at 25 °C.
206views - Open Question
Calculate δs∘rxn for the following reaction. The δs∘ for each species is shown below the reaction. C2H2(g) + 2 H2(g) → C2H6(g) s∘(J/mol⋅K) 200.9 130.7 229.2
300views - Open Question
Calculate ∆S° for NH3(g) + HCl(g) → NH4Cl(s)
390views - Open Question
Calculate ∆S° for C3H4(g) + 2 H2(g) → C3H8(g).
222views - Multiple ChoiceA reaction has ΔrH = -107 kJ mol^-1 and ΔrS = 285 J K^-1 mol^-1. At what temperature is the change in entropy for the reaction equal to the change in entropy for the surroundings?7views
- Multiple ChoiceCalculate ΔS°rxn for the reaction: 2NO(g) + O2(g) → 2NO2(g) given the standard molar entropies: S°(NO) = 210.7 J/mol·K, S°(O2) = 205.0 J/mol·K, S°(NO2) = 240.1 J/mol·K.6views
- Multiple ChoiceCalculate ∆S_rxn for the reaction 2NO(g) + O2(g) → 2NO2(g) given the following standard molar entropies: S°(NO) = 210 J/mol·K, S°(O2) = 205 J/mol·K, S°(NO2) = 240 J/mol·K.3views