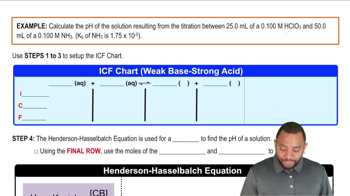
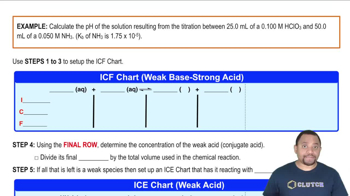
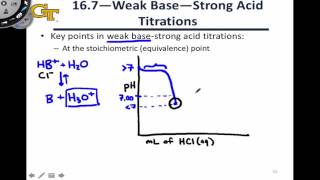
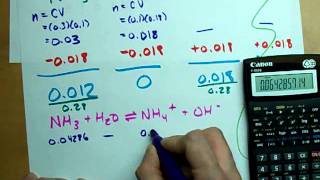18. Aqueous Equilibrium
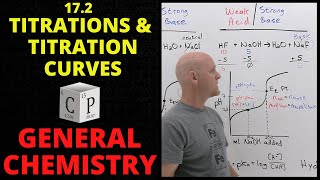
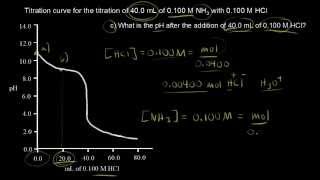
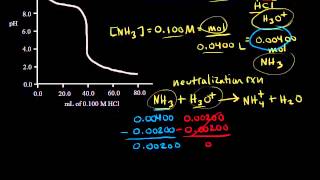
Titrations: Weak Base-Strong Acid
18. Aqueous Equilibrium
Titrations: Weak Base-Strong Acid
Showing 6 of 6 videos
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple ChoiceWhat is the initial pH for the titration of 30.0 mL of 0.850 M NH3 with 0.100 M HCl. Kb for NH3 = 1.8 × 10−5.363views
- Multiple ChoiceThe plot below illustrates which type of titration?
365views - Multiple Choice
Calculate the pH of the solution resulting from the mixing of 75.0 mL of 0.100 M NaC2H3O2 and 75.0 mL of 0.30 M HC2H3O2 with 0.0040 moles of HBr.
388views2rank1comments - Multiple Choice
In order to create a buffer 7.321 g of potassium lactate is mix with 550.0 mL of 0.328 M lactic acid, HC3H5O3. What is the pH of the buffer solution after the addition of 300.0 mL of 0.100 M hydrobromic acid, HBr? The Ka of HC3H5O3 is 1.4 × 10−4.
296views2rank - Open Question
What quantity of NAG3 was required to reach the equivalence point in the titration?
223views