18. Aqueous Equilibrium
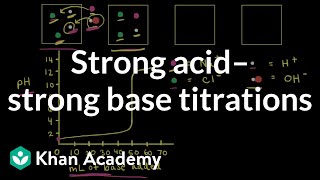
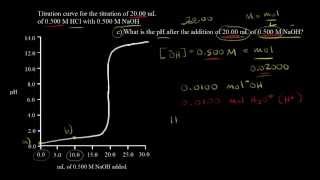
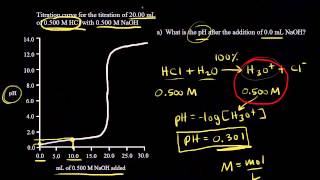
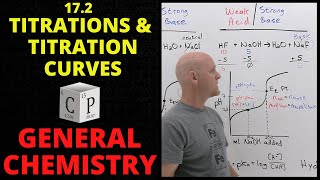
Titrations: Strong Acid-Strong Base
Learn with other creators
Practice this topic
- Multiple Choice
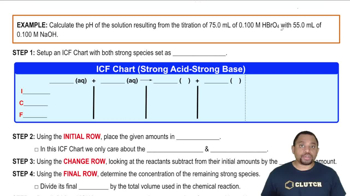
Calculate the pH of the solution resulting from the mixing of 175.0 mL of 0.250 M HNO3 with 75.0 mL of 0.200M Ba(OH)2.
1542views1rank4comments - Multiple Choice
Calculate the pH of the solution resulting from the mixing of 175.0 mL of 0.250 M HNO3 with 75.0 mL of 0.200 M Ba(OH)2.
354views2rank - Multiple Choice
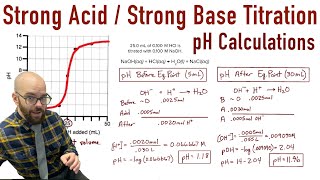
Calculate the pH of the solution resulting from the titration of 110.0 mL of 0.300 M HCl with 330.0 mL of 0.100 M LiOH.
344views3rank - Open Question
A 4.36-g sample of an unknown alkali metal hydroxide is dissolved in 100.0 ml of water. An acid-base indicator is added and the resulting solution is titrated with 2.50 mL of HCl (aq) solution. The indicator changes color signaling that the equivalence point has been reached after 17.0 ml of the hydrochloric acid solution has been added.
169views - Open Question
A solution prepared by mixing 10 mL of 1 M HCl and 10 mL of 1.2 M NaOH has a ph of
222views - Open QuestionCalculate the ph during the titration of 40.00 ml of 0.1000 m hcl with 0.1000 m naoh solution after the following additions of base:237views
- Multiple ChoiceA 100.0 mL sample of 0.18 M HClO4 is titrated with 0.27 M LiOH. Determine the pH of the solution after the addition of 30.0 mL of LiOH.