18. Aqueous Equilibrium
Titrations: Diprotic & Polyprotic Buffers
18. Aqueous Equilibrium
Titrations: Diprotic & Polyprotic Buffers
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
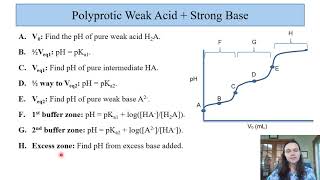
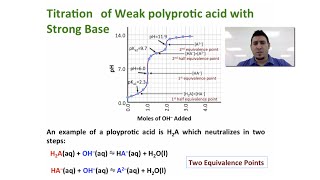
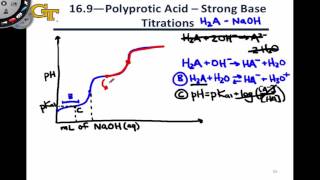
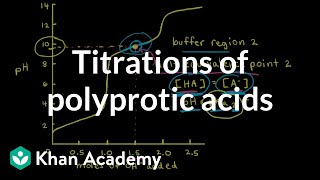
Calculate the pH of 75.0 mL of a 0.10 M of phosphorous acid, H3PO3, when 80.0 mL of 0.15 M NaOH are added. Ka1 = 5.0 × 10−2, Ka2 = 2.0 × 10−7.
308views2rank1comments - Multiple Choice
Find the pH when 100.0 mL of a 0.1 M dibasic compound B (pKb1 = 4.00; pKb2 = 8.00) was titrated with 11 mL of a 1.00 M HCl.
257views2rank - Multiple Choice
Suppose you have 50.1 mL of a H3PO4 solution that you titrate with 15.4 mL of 0.10 M KOH solution to reach the endpoint. What is the concentration of H3PO4 of the original H3PO4 solution?
270views1rank - Open QuestionA 25.0 ml sample of 0.125 moll−1 pyridine (kb=1.7×10−9) is titrated with 0.100 moll−1hcl.166views
- Open Question
What is observed when equal volumes of 0.1 M aqueous HCl and 0.01 M aqueous Na2SO3 are mixed?
220views - Open Question
What is the identity of the acid (H3X) identified via titration of Na3X with HCl?
241views - Multiple Choice30.00 mL of a strong, diprotic acid are titrated with 0.1432 M NaOH. If it takes 24.15 mL to reach the endpoint of the titration, what is the concentration of the acid?