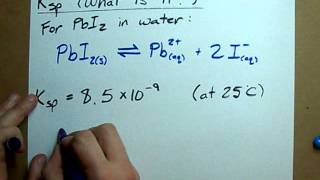18. Aqueous Equilibrium
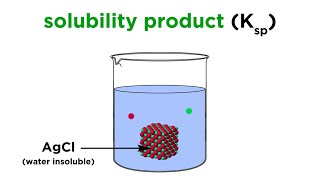
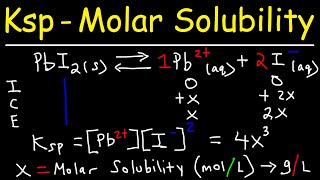
Solubility Product Constant: Ksp
18. Aqueous Equilibrium
Solubility Product Constant: Ksp
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple ChoiceDetermine the molar solubility of CaCO3 in a solution of 0.500 M Na2CO3. Ksp for CaCO3 is 4.96 × 10−9.665views
- Multiple ChoiceWhat is the molar solubility of PbBr2 in pure water? Ksp for PbBr2 is 4.67 × 10−6.932views
- Multiple Choice
Solubility of Sn(OH)2 was found to be 1.11 x 10-9 M; calculate Ksp of this compound.
546views6rank - Multiple Choice
If a saturated solution of Ag2CO3 contains 2.56 × 10−4 M of Ag+ ions, determine its solubility product constant.
398views3rank1comments - Open Question
Calcium oxalate, CaC2O4 (m = 128.1), dissolves to the extent of 0.67 mg L–1 . What is its Ksp?
202views - Open Question
What is the solubility of La(IO3)3 in water? (Ksp of La(IO3)3₃ is 7.5 × 10-12)
227views - Open Question
Which of the following is the correct expression for Ksp for Zn3(PO4)2?
173views - Open Question
Which of the following is the correct expression for Ksp for Zn3(PO4)2?
187views