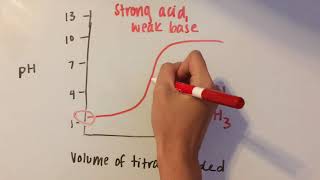
18. Aqueous Equilibrium
Intro to Acid-Base Titration Curves
18. Aqueous Equilibrium
Intro to Acid-Base Titration Curves
Showing 5 of 5 videos
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
What will be the color of the indicator in the above question in a solution that has a pH of 6?
632views1rank4comments - Multiple Choice
Consider the titration of 60.0 mL of 0.200 M H3PO3 solution with 0.350 M potassium hydroxide, KOH solution. How many milliliters of base would be required to reach each of its equivalence points?
998views4rank4comments - Multiple ChoiceWhich of the following acids would be needed to combine with its potassium salt to produce a solution buffered at pH 5.00?
Chlorous acid: pKa = 1.95
Nitrous acid: pKa = 3.34
Formic acid: pKa = 3.74
Acetic acid: pKa = 4.74
Hypochlorous acid: pKa = 7.54308views - Multiple Choice
Consider the titration of 100.0 mL of 0.40 M HCl with 0.40 M NaOH. If sodium hydroxide is the titrant, which volume would place it in excess?
a) 70.0 mL b) 25.0 mL c) 100.0 mL d) 110.0 mL e) 9.0 mL
345views2rank