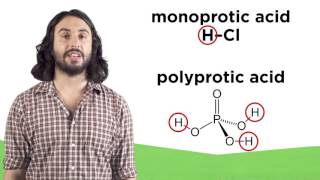
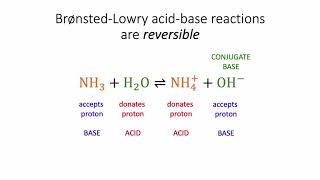17. Acid and Base Equilibrium
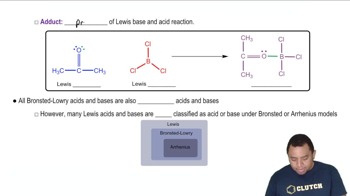
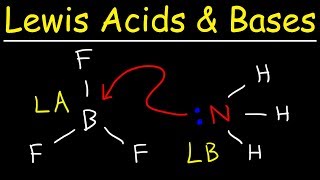
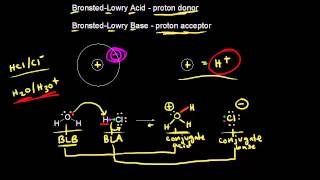
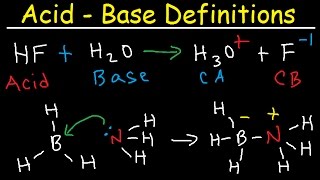
Lewis Acids and Bases
17. Acid and Base Equilibrium
Lewis Acids and Bases
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
Identify the Lewis acids and bases in the following reactions.
a) H+ + OH– ⇌ H2O
b) Cl– + BCl3 ⇋ BCl4–
c) SO3 + H2O ⇌ H2SO4
3914views4rank7comments - Multiple Choice
Identify each of the following compounds as either a Lewis acid, a Lewis base or neither.
a) ZnCl2 b) CN –
c) NH4+ d) Co3+
1582views2rank3comments - Multiple ChoiceWhich of the following illustrates a conjugate acid–base pair?528views
- Multiple Choice
Identify the Lewis acid and Lewis base in the following reaction.
CaO (s) + CO2 (g) → CaCO3 (g)
464views2rank - Open Question
Which of these definitions could be used to define SO2 as a base? Check all that apply.
180views - Open Question
Which term describes a substance that increases the concentration of hydroxide ions in solution?
192views