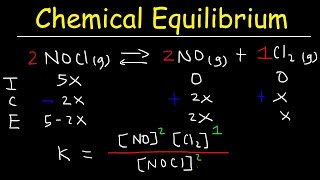
16. Chemical Equilibrium
Intro to Chemical Equilibrium
Learn with other creators
Practice this topic
- Multiple Choice
Which one of the following statements does not describe the equilibrium state?
a. While at equilibrium, a dynamic process is still occurring.
b. The concentration of the reactants is equal to the concentration of the products.
c. The concentration of the reactants and products reach a constant level.
d. At equilibrium, the net concentration of all species is not changing.
e. All are true.
3448views3rank2comments - Multiple Choice
State which is greater in amount:reactants or products, based on the given equilibrium constant, K.
1865views7rank1comments - Multiple Choice
The decomposition of nitrogen monoxide can be achieved under high temperatures to create the products of nitrogen and oxygen gas.
6 NO(aq) ⇌ 3 N2(aq) + 3 O2(aq)
a) What is the equilibrium equation for the reaction above?
b) What is the equilibrium expression for the reverse reaction.
2132views6rank4comments - Multiple Choice
The equilibrium constant, K, for 2 NO (g) + O2 (g) ⇌ 2 NO2 (g) is 6.9 x 102.
What is the [NO] in an equilibrium mixture of gaseous NO, O2, and NO2 at 500 K that contains 1.5 x 10 –2 M O2 and 4.3 x 10 –3 M NO2?
4892views13rank15comments - Open Question
Which statement correctly describes a reaction in dynamic equilibrium? At dynamic equilibrium, the reactions stop and the amounts of reactants and products do not change. At dynamic equilibrium, the reactions continue but the amounts of reactants and products do not change. At dynamic equilibrium, the reactions stop but the amounts of reactants and products are changing. At dynamic equilibrium, the reactions continue and the amounts of reactants and products are changing.
1237views