Half-Life
15. Chemical Kinetics / Half-Life / Problem 2
The first-order reaction of A molecules (gray spheres) occurs in three vessels, 1, 2, and 3, of equal volume.
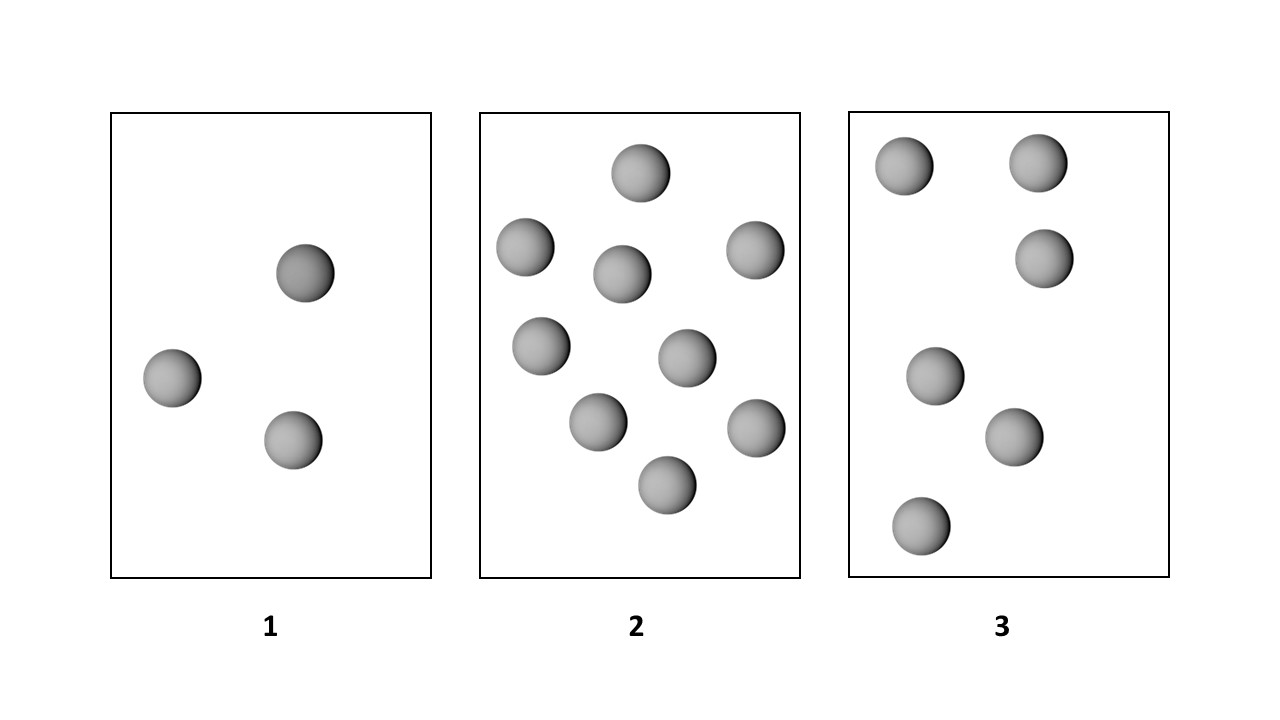 If the volume of each vessel is increased by a factor of 2, what will happen to the rates and half-lives of the reaction?
If the volume of each vessel is increased by a factor of 2, what will happen to the rates and half-lives of the reaction?
Was this helpful?

Learn this concept