Please be aware that you practice a sample exam set, which means that it’s mimicking a real exam. In order to have more accurate experience:
or
- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

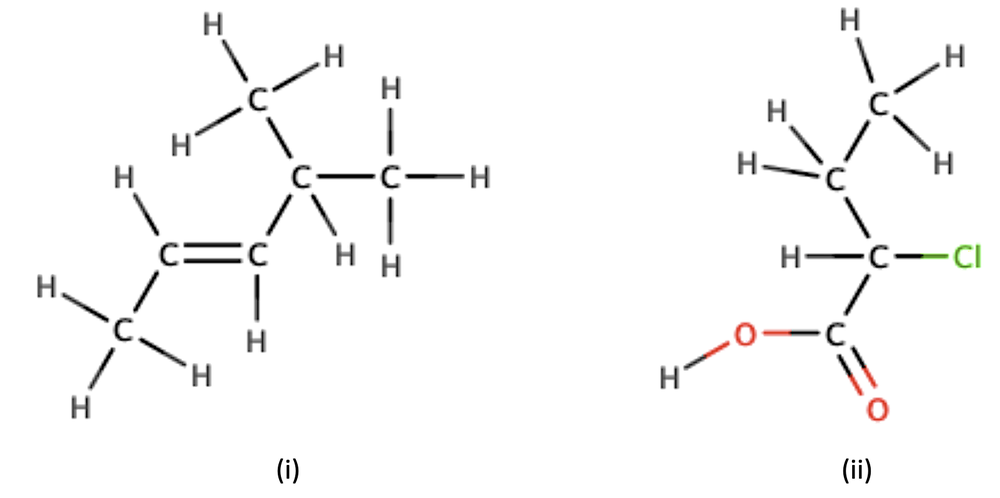
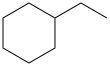
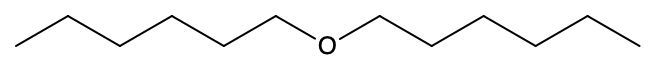
Draw the condensed formula for the following structural formulas
Saccharin (C7H5NO3S) is an artificial sweetener that is about 550 times as sweet as sucrose. Each carbon, oxygen, and nitrogen atom follow the octet rule, while each hydrogen only forms one bond. The sequence of carbon atoms is shown below. Provide the complete structural formula for saccharin.

Glucose is an interesting molecule. It can exist as an open-chain molecule or as any of its four cyclic isomers. One of its cyclic isomers is shown below. What do the dashed bonds represent in this structure?

Identify the orbitals that overlap for each of the indicated bonds below:

Are the molecules shown below enantiomers or identical?

Is optical isomerism present in the following compound?

Identify the molecule below that is most soluble in benzene or pentane.
Acetic acid (CH3CO2H) or Octanoic acid (CH3(CH2)6CO2H)
Provide the structure of an alkane with the name 4-ethyl-4-methylheptane.
Which of the structures below represent an isobutyl group

Give the IUPAC name for the compound below with the molecular formula of C10H22

Give the name of the compound:
1,3-dibromocyclobutane has two stereoisomers, each of which has different chemical and physical properties. How many stereoisomers are possible for 1,2-dibromocyclobutane? Are any of them nonpolar?
Which of the following molecules contains 4 carbons?
Methyl methanoate, 1-ethoxybutane, 1-propanol, and 2-pentanone
Which of the following is the correct name for the alkene?

Which of the following is the correct name for the alkyne?

Draw the structure of hexan-3-one.
What is the structure of butanal, an aldehyde?
Provide the name of the following carboxylic acid.

Which of the following molecules contains a carbonyl group? Methyl methanoate, 1-ethoxybutane, 1-propanol, and 2-pentanone.
Write the name of the given ether.
Provide the structure of sec-butylamine.
Determine the systematic name of the given disubstituted benzene.
What is/are the product/s for the following alkane substitution if only monosubstitution occurred?

What makes high-density polyethylene denser than low-density polyethylene based on their molecular structure?
Identify the organic product of the addition reaction below: