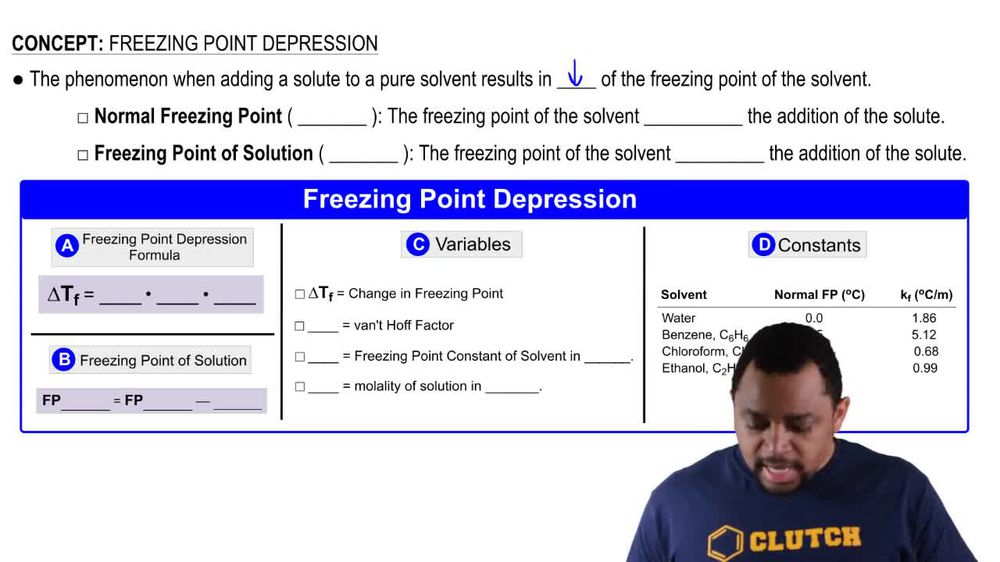
Freezing Point Depression
14. Solutions / Freezing Point Depression / Problem 2
Problem 2
When 9.5 g of lithium stearate (LiC18H35O2, MW = 290.47 g/mol) is dissolved in 550 g benzene, the resulting solution freezes at 0.30 °C lower than pure benzene (FP = 5.5 °C). Calculate the value of Kf (freezing point constant) for benzene.

Learn this concept
Freezing Point Depression
Take your learning anywhere!
Prep for your exams on the go with video lessons and practice problems in our mobile app.
