- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Identify which among the given below is a state function and which is not.
(a) The amount of work done to compress a gas.
(b) The pressure inside a scuba tank.
(c) The mass of a standard gold bar: 12.4 kg.
Consider a process that results in the following volume and temperature change:

Is there any work done? If yes, identify the sign of the work done.
The refrigerant used in air-conditioning systems can easily undergo vaporization at atmospheric pressure and compression at higher pressures. The system then acts as a closed system consisting of the refrigerant undergoing these two stages. The vaporization occurs in an expansion chamber at low pressures. The compression of the vapor back to its liquid phase occurs in a compression chamber at high pressure.
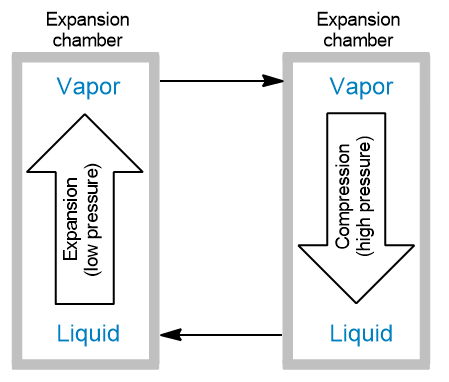
Which of the following statements is true based on this information?
Consider the mixing tank below:

The flow of the two inlets is balanced such that the solution inside the tank and the solution that flows through the outlet has a constant composition. The temperature is also maintained through the use of a heating mechanism. If the inlet and outlet were blocked, what type of system will the tank be?
Which of the following represent q and w for a system that is doing work on the surroundings and losing heat to surroundings?
a) q > 0, w > 0
b) q > 0, w < 0
c) q = 0, w > 0
d) q < 0, w = 0
e) q < 0, w < 0
f) q < 0, w > 0
Which of the following is the best interpretation of the First Law of Thermodynamics?
a) The total work of the universe is decreasing.
b) The total mass of the universe is increasing.
c) The total energy of the universe is constant.
d) The total heat of the universe is decreasing.
e) The total enthalpy of the universe is increasing.
f) The total entropy of the universe is constant.