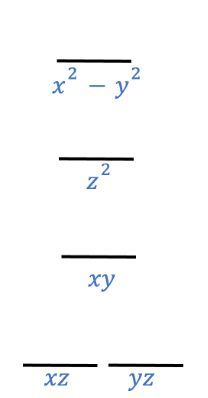- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Consider the following substitution reaction of the complex trans-[RuCl2(bipy)2]:
trans-[RuCl2(bipy)2](aq) + NO2–(aq) → trans-[RuCl(bipy)2(NO2)] + Cl–(aq)
Predict the crystal field splitting diagram for the reactant trans-[RuCl2(bipy)2] given that Cl– is a much more weaker-field ligand than bipyridine (bipy).
Ni2+ can form tetrahedral and square planar complexes having a formula of NiX2L2, where X is Br– or CN– and L is the monodentate ligand triphenylphosphine (PPh3). Give the possible structures for each NiX2L2 complex and identify which will have a net dipole moment, considering that NiBr2L2 is paramagnetic and Ni(CN)2L2 is diamagnetic.
The crystal field energy-level diagram for a square pyramidal complex (ML5) is known to be intermediate between an octahedral complex (ML6) and a square planar complex (ML4).
Does the crystal field energy-level diagram below correspond to a square pyramidal, ML5, complex with one ligand along the ±z-axis, two along the ±x-axis, and two along the ±y-axis?
Cobalt complexes, characterized by the chemical formula CoX2L2, wherein X is Cl− or N-bonded NCS− and L is a monodentate triphenylphosphine ligand, can exhibit either a tetrahedral or square planar geometry. What are the crystal field energy-level diagrams that illustrate the distribution of electrons within the orbitals for a tetrahedral and square-planar CoX2L2 complex?