- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Which of the following statements explain why the pressure of a gas in an isolated container (with a fixed volume) can increase over time at constant temperature?
Determine whether the atoms of 0.5 L of oxygen gas and 0.5 L of nitrogen gas have the same average kinetic energy at room temperature and atmospheric pressure.
Find the kinetic energy of 1.00 mole of the following noble gases at 25 °C: He, Ne, and Ar.
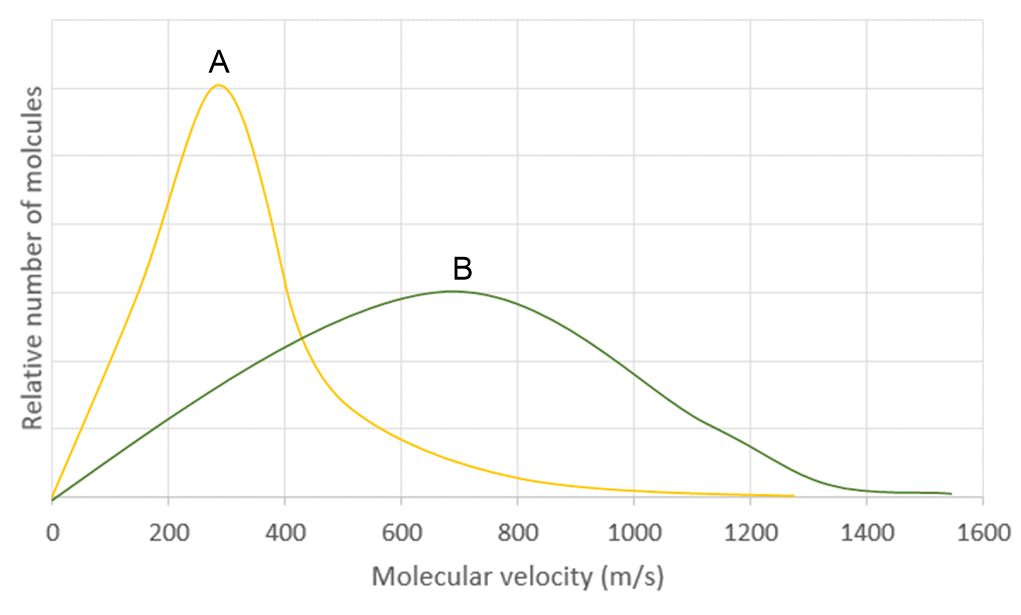
Which of the following statement is true for the image shown where gas A and B are different gases?

a. The root-mean-square speed is the highest speed for both gas A and B.
b. The most probable speed is the highest speed for gas B only.
c. The root-mean-square speed is the highest speed for gas B only.
d. The average speed is the highest speed for gas A only.
e. None of the above
A certain amount of Br2 gas is placed inside a container at 550 K. Determine the most probable speed of the molecules at this temperature.
Consider the graph below comparing the velocity distribution of PH3 and Kr at the same temperature. Identify which curve represents a Kr.
Consider the graph below comparing the velocity distribution of the same molecule at different temperatures. Identify which curve represents a lower temperature.

Legend:
Curve A → blue
Curve B → red
Which conditions increases the likelihood of a gas deviating from ideal behavior?
I. low pressure or high temperature
II. polar molecules
III. nonpolar molecules
IV. high pressure or low temperature
Identify which statements describe a gas that is placed at a very high temperature.
i) The intermolecular forces become more relevant to its behavior
ii) The average kinetic energy increases
iii) Particle collisions increase
Consider the phase diagram of CO2 below:

Identify at which labeled point is CO2 most likely to behave as an ideal gas.
Which one of the following four gases is expected to have the lowest total molecular volume compared to the total occupied volume?
Identify the correct statements below:
i) van der Waals constant "b" accounts for the attractive forces between the molecules and "a" accounts for the volume occupied by the gas molecules
ii) The value of van der Waals constant "a" depends on temperature and "b" depends on pressure.
iii) The value of van der Waals constants "a" depends on the identity of gas while "b" is constant for all gases.
iv) The value of van der Waals constant "b" depends on temperature and "a" depends on pressure.
Calculate the pressure in a 5000 L industrial tank containing 322.4 kg of oxygen at 25.0°C using the van der Waals equation.