10. Periodic Properties of the Elements - Part 2 of 3
10. Periodic Properties of the Elements / Periodic Trend: Ionic Radius / Problem 13
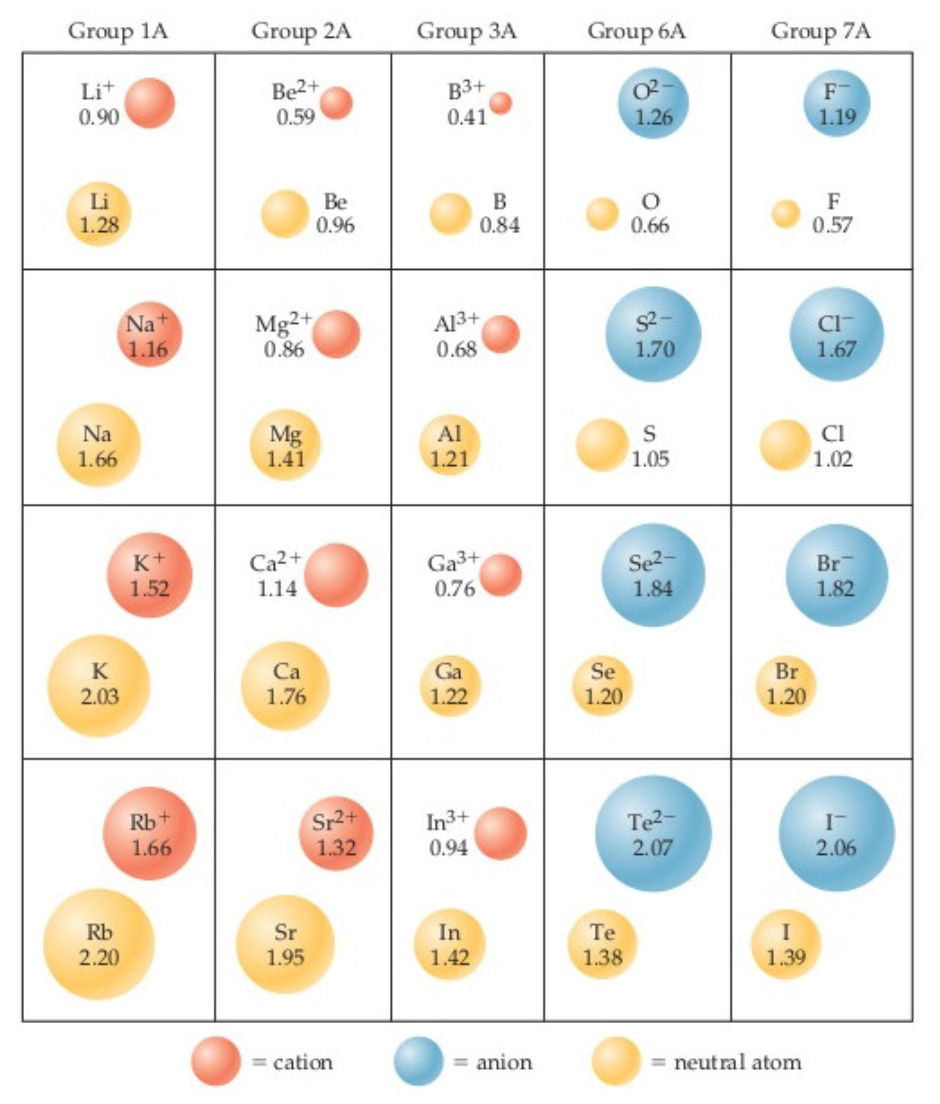
Calculate the differences between the actual and theoretical cation-anion distances for the following ionic compounds:LiCl, NaF, KBr, RbI
The experimentally measured cation-anion distances are: LiCl = 2.50 Å; NaF = 2.31 Å; KBr = 3.30 Å and RbI = 3.67 Å
Use the diagram below to determine the theoretical cation-anion distances for the ionic compounds above.

Learn this concept