Here are the essential concepts you must grasp in order to answer the question correctly.
Covalent Bonding
Covalent bonding occurs when two atoms share electrons to achieve a full outer shell, leading to stability. In fructose, each carbon atom forms four covalent bonds, while oxygen forms two and hydrogen forms one. Understanding these bonding patterns is crucial for drawing the structural formula accurately.
Recommended video:
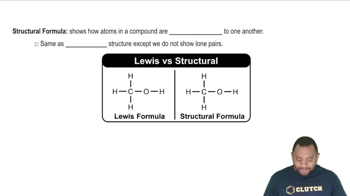
Structural Formula
The structural formula of a compound illustrates the arrangement of atoms and the bonds between them. For fructose, this includes the specific connectivity of carbon, hydrogen, and oxygen atoms. Drawing the complete structural formula requires knowledge of how to represent single, double, and triple bonds between atoms.
Recommended video:
Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms in a molecule. In fructose, the geometry is influenced by the tetrahedral shape around carbon atoms and the bent shape around oxygen atoms. Understanding molecular geometry helps in visualizing the spatial orientation of the atoms, which is essential for accurately depicting the structural formula.
Recommended video:
Molecular Geometry with Two Electron Groups

 Verified step by step guidance
Verified step by step guidance