Here are the essential concepts you must grasp in order to answer the question correctly.
Hybridization
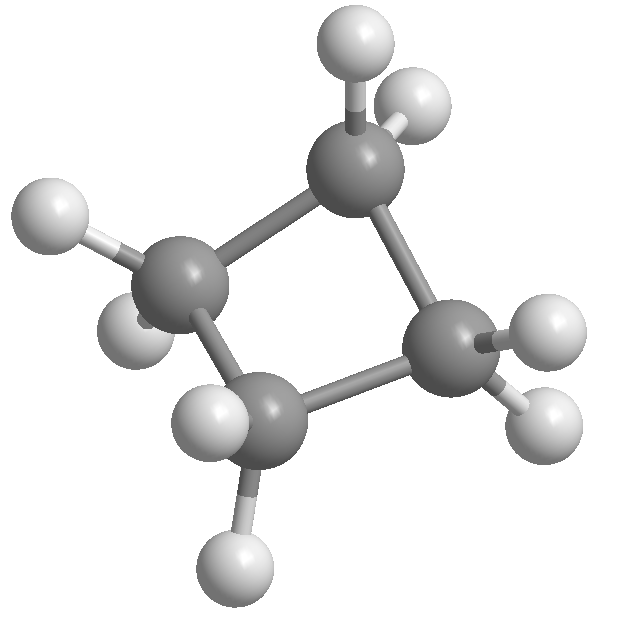
Hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals that can accommodate bonding. In cyclopropane, the carbon atoms undergo sp3 hybridization, resulting in four equivalent hybrid orbitals that form sigma bonds with adjacent atoms. This arrangement leads to a tetrahedral geometry, which is strained in a three-membered ring, contributing to the molecule's reactivity.
Recommended video:
Ring Strain
Ring strain occurs in cyclic compounds when the bond angles deviate from the ideal tetrahedral angle of 109.5 degrees. In cyclopropane, the three-membered ring forces the bond angles to approximately 60 degrees, creating significant angle strain. This strain makes cyclopropane more reactive than larger cyclic hydrocarbons, as the molecule seeks to relieve this strain through chemical reactions.
Recommended video:
Reactivity of Alkenes
The reactivity of alkenes is largely due to the presence of a carbon-carbon double bond, which is more reactive than single bonds. In the thermal decomposition of cyclopropane to propene, the formation of propene involves breaking the strained cyclopropane structure and forming a more stable alkene. The transition from a strained cyclic structure to a more stable linear alkene contributes to the overall reactivity of cyclopropane.
Recommended video: