What is the coordination number of the metal in each of the following complexes?
(d) [ZrF8]4-
(e) [Fe(EDTA)(H2O)]-
 Verified step by step guidance
Verified step by step guidance

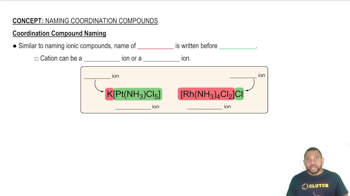

What is the coordination number of the metal in each of the following complexes?
(d) [ZrF8]4-
(e) [Fe(EDTA)(H2O)]-
Identify the oxidation state of the metal in each of the following compounds.
(a) Co(NH3)3(NO2)3
(b) [Ag(NH3)2]NO3
(c) K3[Cr(C2O4)2Cl2]
(d) Cs[CuCl2]
What is the formula, including the charge, for each of the following complexes?
(a) An iridium(III) complex with three ammonia and three chloride ligands
(b) A chromium(III) complex with two water and two oxalate ligands
(c) A platinum(IV) complex with two ethylenediamine and two thiocyanate ligands
What is the formula, including the charge, for each of the following complexes?
(a) An iron(III) complex with six water ligands
(b) A nickel(II) complex with two ethylenediamine and two bromide ligands
(c) A platinum(II) complex with two chloride and two ammonia ligands
Draw the structure of the iron oxalate complex [Fe(C2O4)3]3-. Describe the coordination geometry, and identify any chelate rings. What are the coordination number and the oxidation number of the iron?